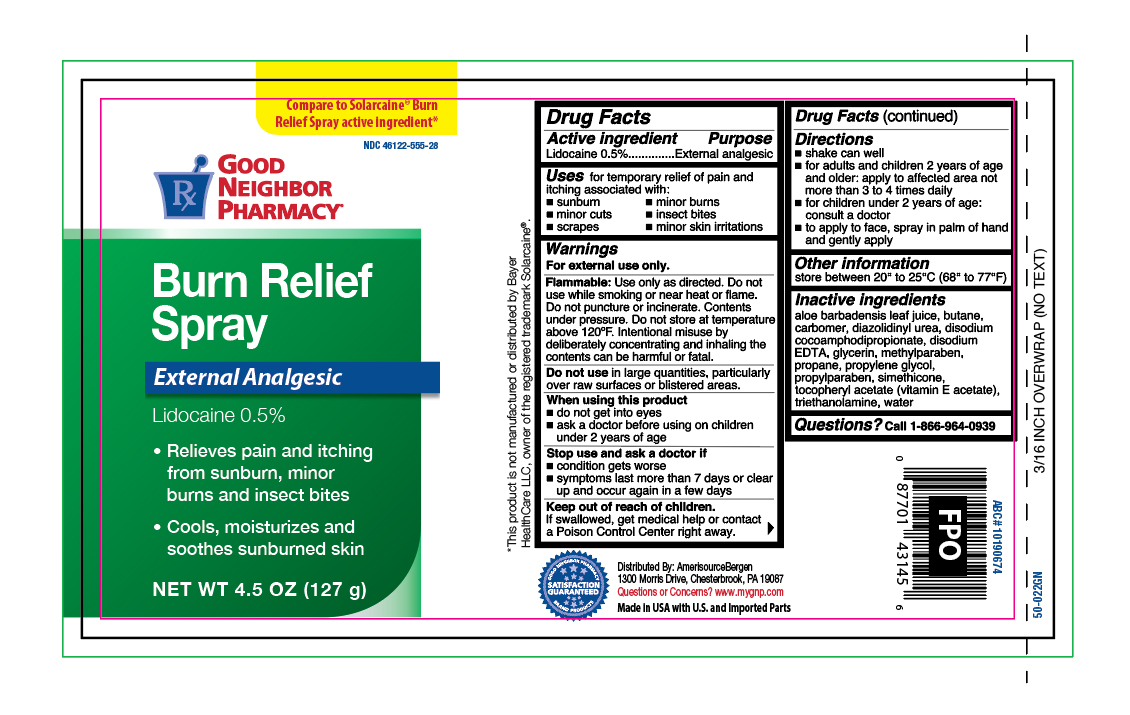
Active Ingredients
Lidocaine 0.5%
Purpose
External analgesic
Uses
for the temporary relief of pain and itching associated with:
- sunburn
- minor burns
- minor cuts
- scrapes
- insect bites
- minor skin irritations
Warnings
For external use only.
Flammable:
Do not use while smoking or near heat or flame. Do not puncture or incinerate. Contents under pressure. Do not store at temperature above 120ºF. Intentional misuse by delibrately concentrating and inhaling the contents can be harmful or fatal.
Do not use
in large quantities, particularly over raw surfaces or blistered areas.
When using this product
- do not get into eyes
- ask a doctor before using on children under 2 years of age
Stop use and ask a doctor if
- condition gets worse
- symptoms last more than 7 days or clears up and occurs again in a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake can well
- for adults and children 2 years and older, apply to affected area not more than 3 to 4 times daily
- for children under 2 years of age, ask a doctor
- to apply to face spray in palm of hand and gently apply
Other information
store between 20º and 25ºC (68º and 77ºF)
Inactive ingredients
aloe barbadensis leaf juice, butane, carbomer, diazolidinyl urea, disodium cocoamphodipropionate, disodium EDTA, glycerine, methylparaben, propane, propylene glycol, propylparaben, tocopheryl acetate (vitamin E acetate), triethanolamine, simethicone, water
Questions?
Call 1-866-964-0939
Principal Display Panel
GOOD
NEIGHBOR
PHARMACY
Burn Relief
Spray
External Analgesic
Lidocaine 0.5%
- Relieves pain and itching from sunburn, minor burns and insect bites
- Cools, moisturizes and soothes sunburned skin
NET WT 4.5 OZ (127 g)