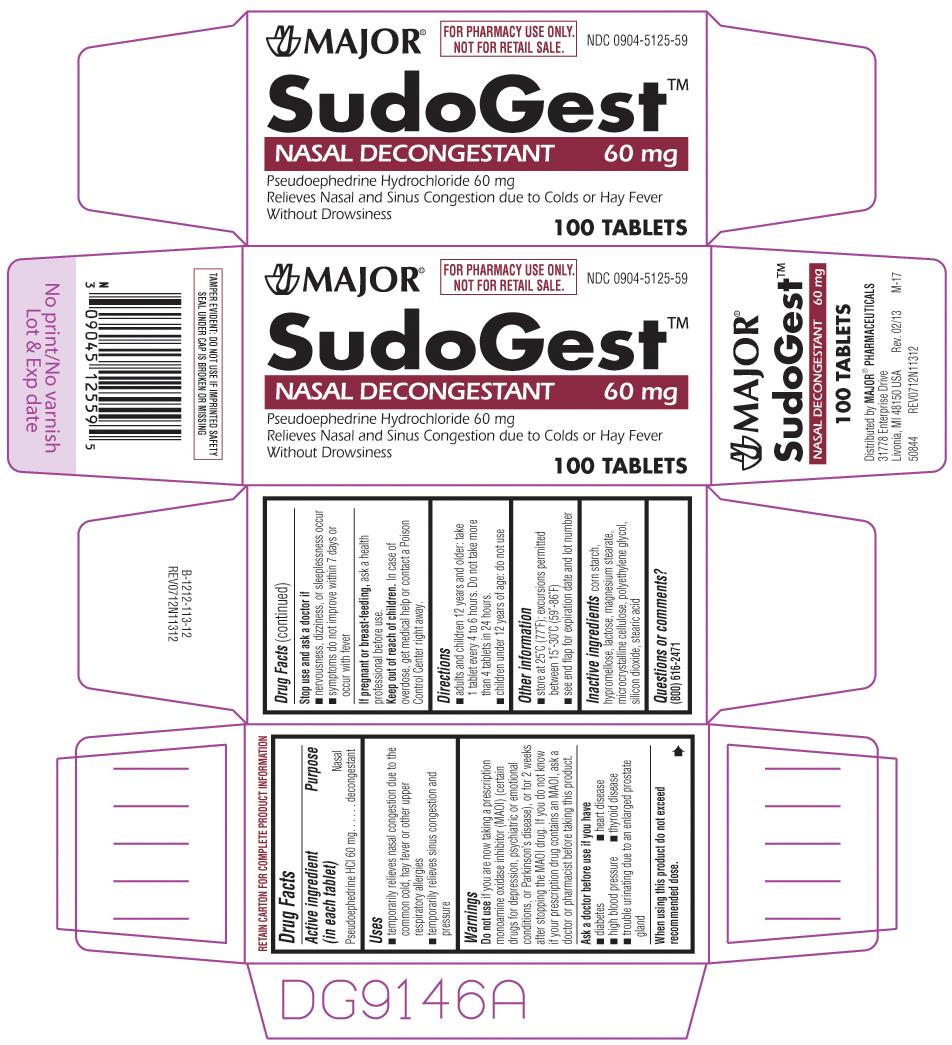
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- diabetes
- thyroid disease
- heart disease
- high blood pressure
- trouble urinating due to enlargement of the prostate gland
Directions
- adults and children 12 years and older: take 1 tablet every 4 to 6 hours. Do not take more than 4 tablets in 24 hours.
- children under 12 years of age: do not use
Other information
- store at 25ºC (77ºF); excursions permitted between 15˚-30˚C (59˚-86˚F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, stearic acid
Principal display panel
MAJOR®
FOR PHARMACY USE ONLY.
NOT FOR RETAIL SALE.
NDC 0904-5125-59
SudoGest™
NASAL DECONGESTANT 60 mg
Pseudoephedrine Hydrochloride 60 mg
Relieves Nasal and Sinus Congestion due to Colds or Hay Fever
Without Drowsiness
100 TABLETS
Distributed by MAJOR® PHARMACEUTICALS
31778 Enterprise Drive
Livonia, MI 48150 USA Rev. 01/13 M-17
50844 REV0712N11312
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Major 44-113