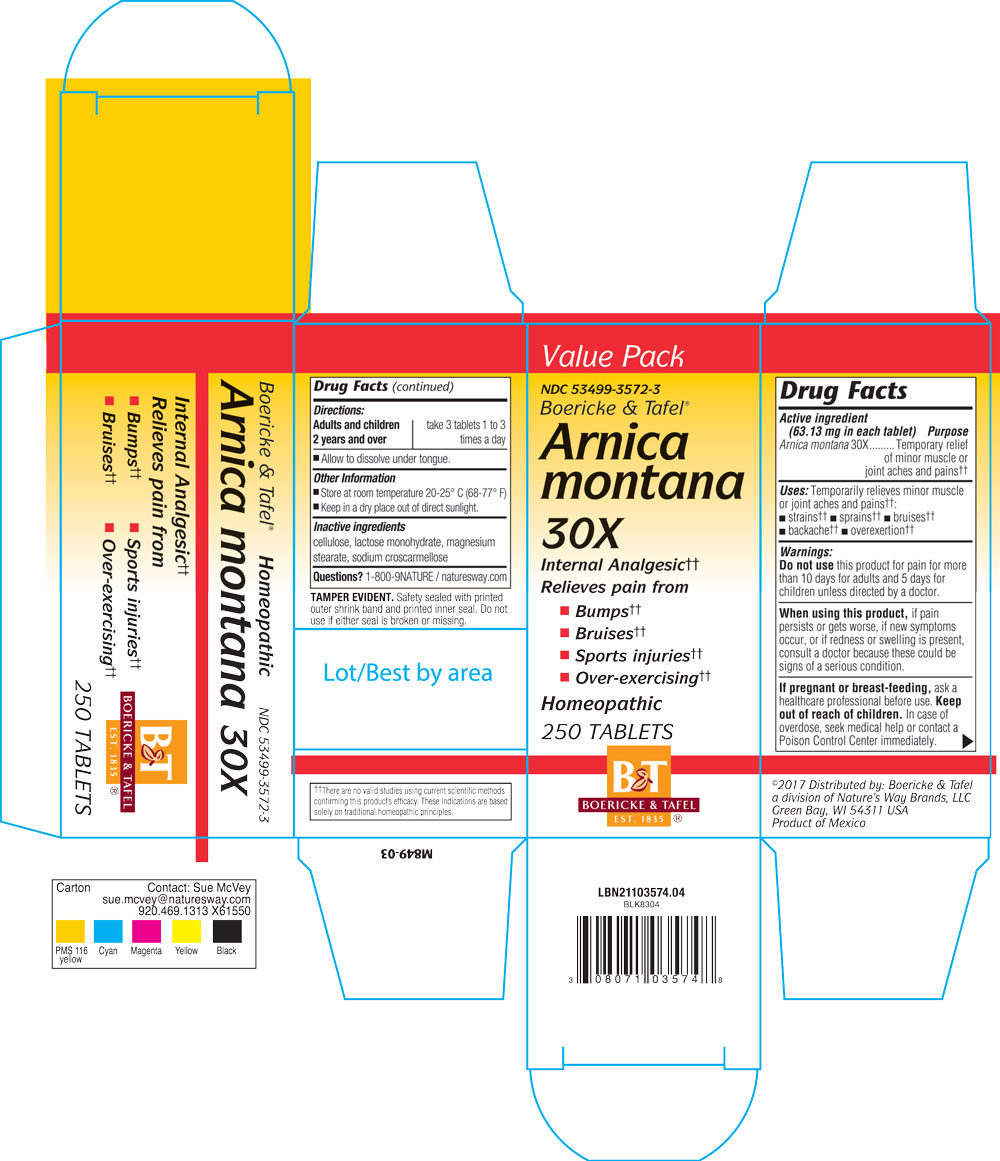
Dosage & Admininstration
Adults or children over 2 years: Take 3 tablets 1 to 3 times per day.
Allow to dissolve under tongue.
Indications & Usage
Temporarily relieves minor muscle or joint aches and pain: strains, sprains, bruises, backached, overexertion.
Purpose
Temporarily relieves minor muscle or joint aches and pain: strains, sprains, bruises, backached, overexertion.
Warning
Do not use this product for pain for more than 10 days for adults and 5 days for children unless directed by a doctor.
When using
When using this product, if pain persists or gets worse, if new symptoms occur, or if redness or swelling is present, consult a doctor because these could be signs of serious conditions.