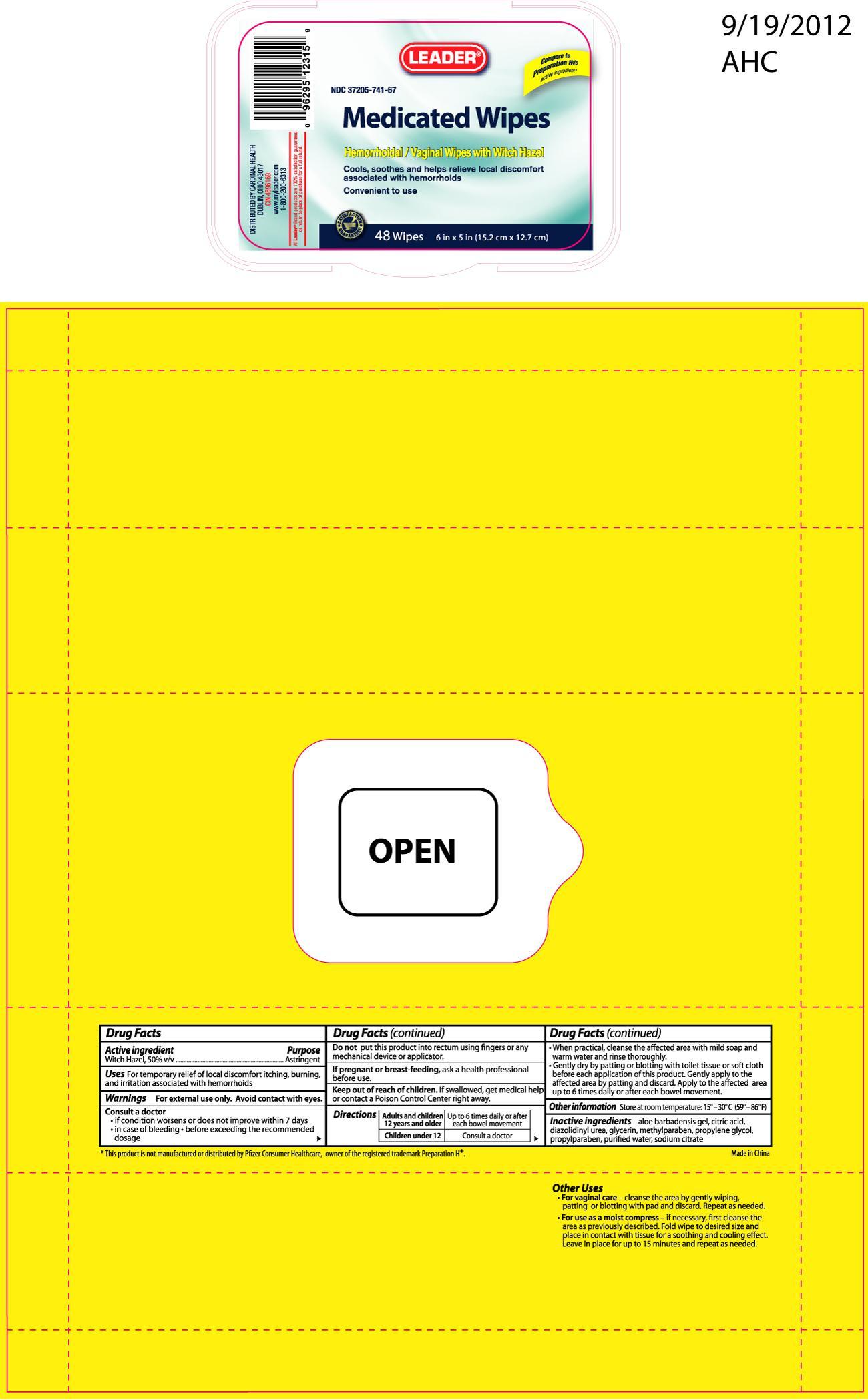
Active ingredient Purpose
Witch Hazel, 50% v/v................................................................Astringent
Uses
For temporary relief of local discomfort itching, burning, and irritation associated with hemorrhoids
Consult a doctor
- if condition worsens or does not improve within 7 days
- in case of bleeding
- before exceeding the recommended dosage
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years and older, up to 6 times daily or after each bowel movement
Children under 12, consult a doctor
When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
Gently dry by patting or blotting with toilet tissue or soft cloth before each application of this product
Gently apply to the affected area by patting and discard
Apply to the affected area up to 6 times daily or after each bowel movement
Inactive ingredients
aloe barbadensis gel, citric acid, diazolidinyl urea, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate