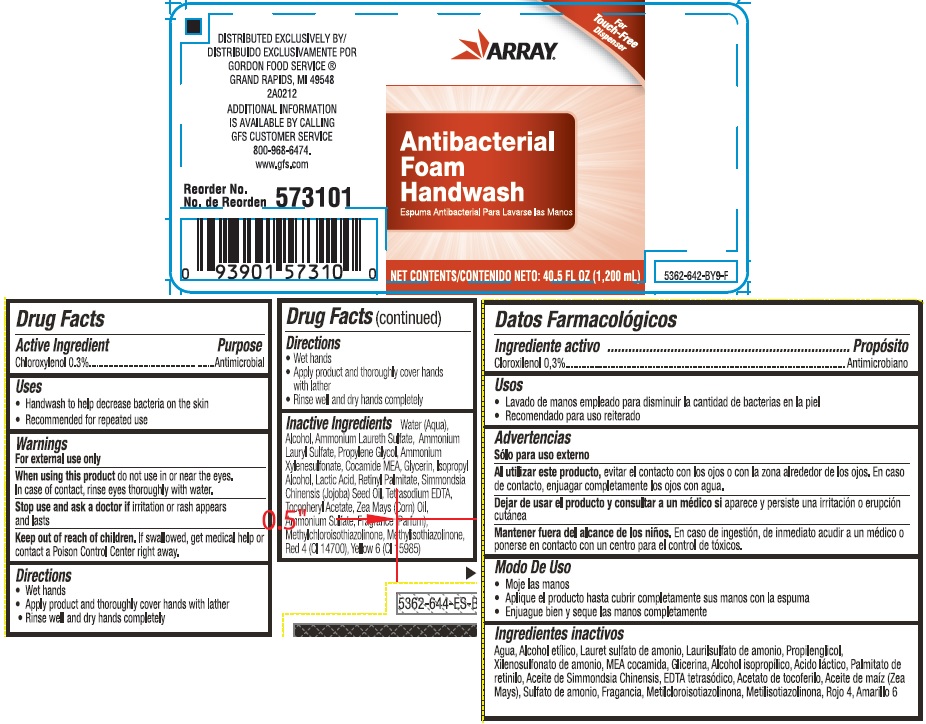
Warnings
For external use only
Directions
- Wet hands
- Apply a small amount of product and work into a lather
- Rinse well and dry hands completely
Inactive ingredients
Water (Aqua), Alcohol, Ammonium Laureth Sulfate, Ammonium Lauryl Sulfate, Propylene Glycol, Ammonium Xylenesulfonate, Cocamide MEA, Glycerin, Isopropyl Alcohol, Lactic Acid, Retinyl Palmitate, Simmondsia Chinensis (Jojoba) Seed Oil, Tetrasodium EDTA, Tocopheryl Acetate, Zea Mays (Corn) Oil, Ammonium Sulfate, Fragrance (Parfum), Methylchloroisothiazolinone, Methylisothiazolinone, Red 4 (CI 14700), Yellow 6 (CI 15985)