GOLDEN FAIR FOUNDATION SPF 15- titanium dioxide, zinc oxide powder
Bare Escentuals Beauty Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
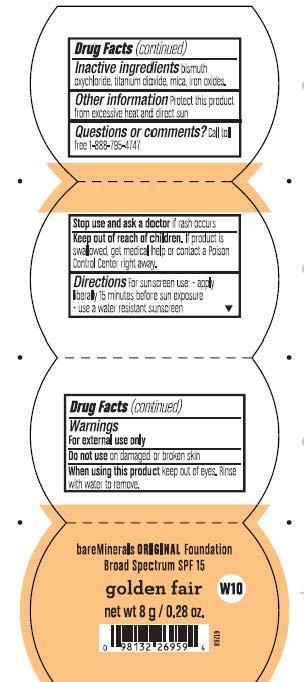
Golden Fair Foundation SPF 15
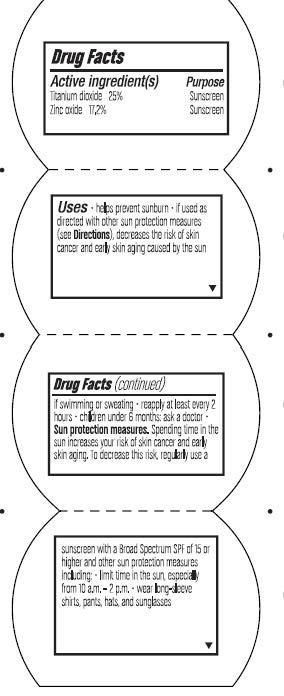
Uses
- helps prevent sunbur
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions for sunscree use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen
- if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
Sun protection measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especiallyfrom 10 a.m. -2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
Warnings
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
| GOLDEN FAIR FOUNDATION SPF 15
titanium dioxide, zinc oxide powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bare Escentuals Beauty Inc. (087008363) |
| Registrant - Ei Inc. (105803274) |