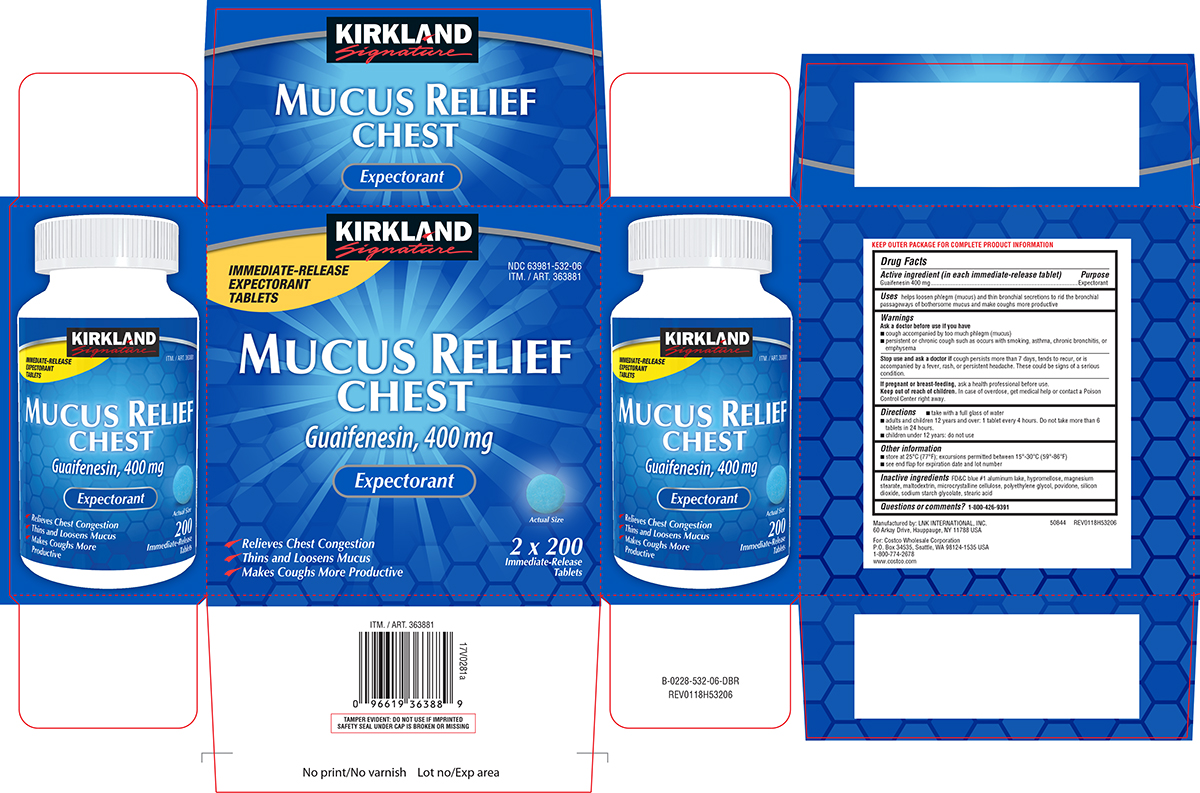
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- cough accompanied by too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- take with a full glass of water
- adults and children 12 years and over: 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours.
- children under 12 years: do not use
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
FD&C blue #1 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic acid
Principal Display Panel
KIRKLAND
Signature
IMMEDIATE-RELEASE
EXPECTORANT
TABLETS
NDC 63981-532-06
ITM. / ART. 363881
MUCUS RELIEF
CHEST
Guaifenesin, 400 mg
Expectorant
Actual Size
√Relieves Chest Congestion
√Thins and Loosens Mucus
√Makes Coughs More Productive
2 x 200
Immediate-Release
Tablets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
50844 REV0118H53206
Manufactured by: LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788 USA
For: Costco Wholesale Corporation
P.O. Box 34535, Seattle, WA 98124-1535 USA
1-800-774-2678
www.costco.com
Kirkland 44-532