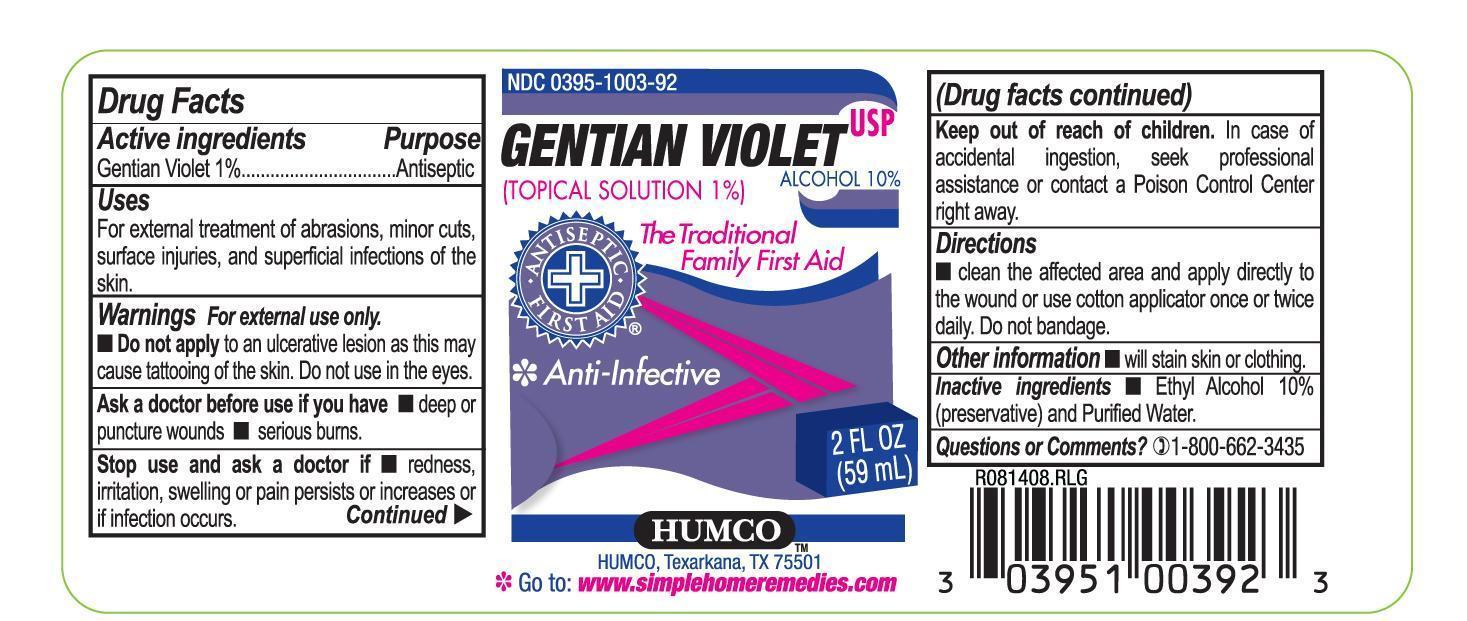
Uses
For external treatment of abrasions, minor cuts, surface injuries, ad superficial infections of the skin.
Warnings
For external use only.
Do not apply to an ulcerative lesion as this may cause tatooing of the skin. Do not use in the eyes.
Ask a doctor before use if
redness, irritation, swelling or pain persists or increases or if infection ocurs.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center right away.