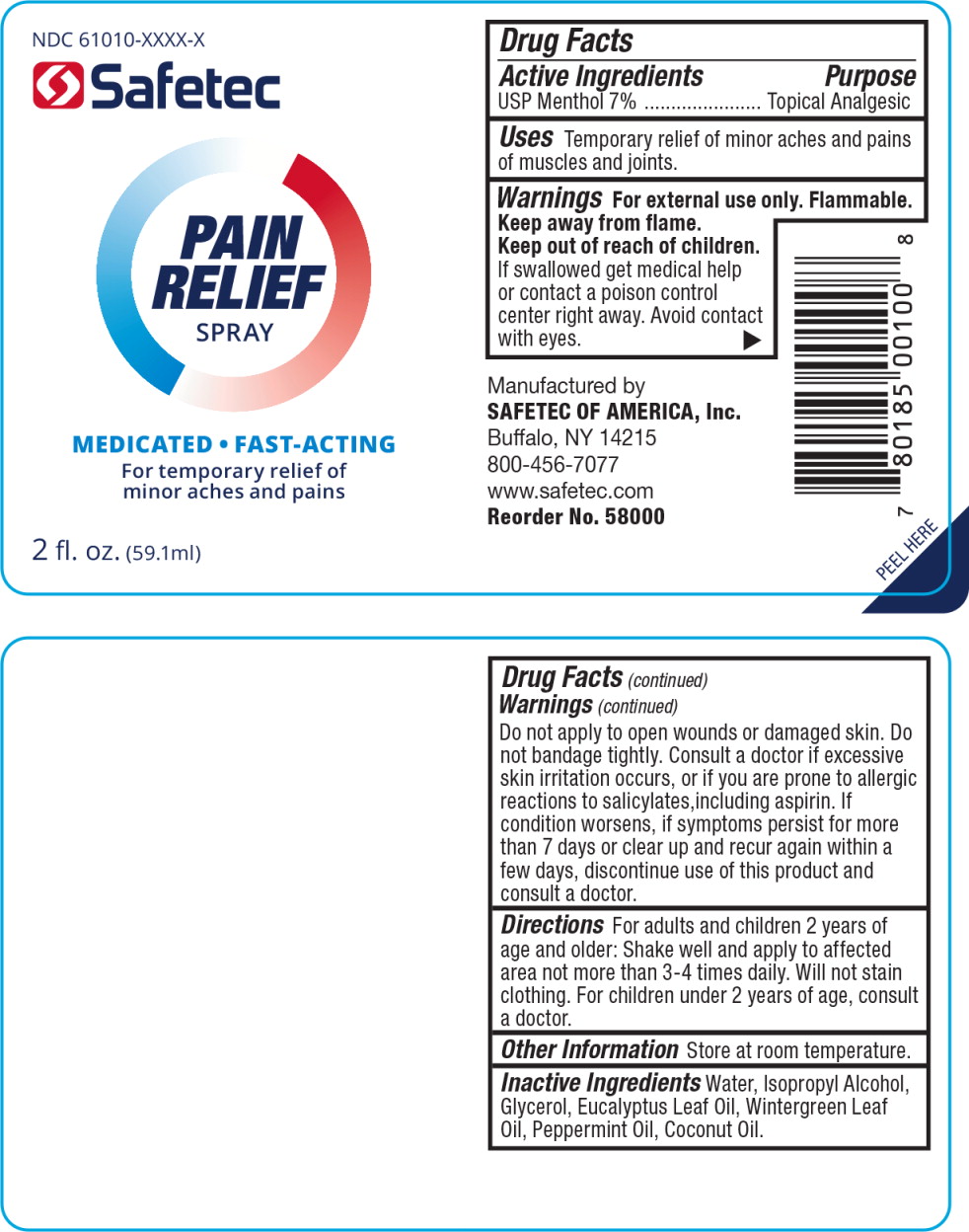
Active Ingredients
USP Menthol 7%
Purpose
Topical Analgesic
Uses
Temporary relief of minor aches and pains of muscles and joints.
Warnings
For external use only.
Flammable.
Keep away from flame.
Keep out of reach of children.
If swallowed get medical help or contact a poison control center right away. Avoid contact with eyes.
Do not apply to open wounds or damaged skin. Do not bandage tightly.
Consult a doctor if excessive skin irritation occurs, or if you are prone to allergic reactions to salicylates,including aspirin. If condition worsens, if symptoms persist for more than 7 days or clear up and recur again within a few days, discontinue use of this product and consult a doctor.
Directions
For adults and children 2 years of age and older: Shake well and apply to affected area not more than 3-4 times daily. Will not stain clothing. For children under 2 years of age, consult a doctor.
Other Information
Store at room temperature.
Inactive Ingredients
Water, Isopropyl Alcohol, Glycerol, Eucalyptus Leaf Oil, Wintergreen Leaf Oil, Peppermint Oil, Coconut Oil.
Principal Display Panel - Pain Relief Spray Bottle Label
NDC 61010-XXXX-X
Safetec
PAIN
RELIEF
SPRAY
MEDICATED FAST-ACTING
For temporary relief of minor aches and pains
2 fl. oz. (59.1 ml)
Safetec of America, Inc.