OXYGEN COMPRESSED LABEL
MEDICAL GAS OXYGEN-2 OXYGEN COMPRESSED USP UN 1072 PRODUCED BY AIR LIQUEFACTION PROCESS
DANGER: HIGH PRESSURE OXIDIZING GAS. VIGOROUSLY ACCELERATES COMBUSTION. STORE AND USE WITH ADEQUATE VENTILATION KEEP OIL AND GREASE AWAY. OPEN VALVE SLOWLY. USE ONLY WITH EQUIPMENT CLEANED FOR OXYGEN SERVICE AND RATED FOR CYLINDER PRESSURE. CLOSE VALVE AFTER EACH USE AND WHEN EMPTY. ALWAYS REPLACE CYLINDER CAP WHEN NOT IN USE. REFER TO ATLANTA MEDICAL GAS MATERIAL SAFETY DATA SHEET.
HEALTH HAZARDS: INHALATION FOR PROLONGED EXPOSURE MAY RESULT IN PNEUMONIA. ELEVATED CONCENTRATIONS PRESENT A RISK OF INFLAMMATION OR ORGANIC MATTER IN BODY. FIRST AID: IF INHALED, REMOVE TO FRESH AIR. IF NOT BREATHING GIVE ARTIFICIAL RESPIRATION, PREFERABLY MOUTH TO MOUTH. CALL A PHYSICIAN IMMEDIATELY AND ADVISE HIM OF EXPOSURE TO OXYGEN.
FOR EMERGENCY USE ONLY WHEN ADMINISTERED BY PROPERLY TRAINED PERSONNEL FOR OXYGEN DEFICIENCY AND RESUSCITATION. FOR ALL OTHER MEDICAL APPLICATIONS Rx ONLY. CAS#7782-44-7 L/333
CONTENTS
CYLINDER TYPE LITERS
T 8688
H 7981
M 3198
E 680
DD 640
D 413
C 248
BB 170
B 170
AA 113
AAA 34

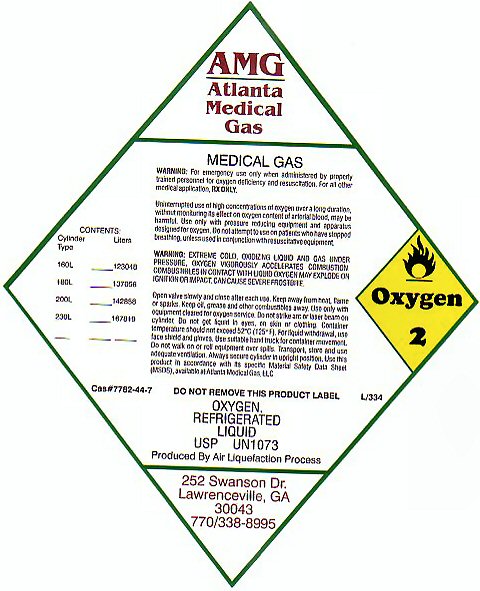
OXYGEN REFRIGERATED LIQUID
MEDICAL GAS OXYGEN-2 OXYGEN REFRIGERATED LIQUID USP UN1073 PRODUCED BY AIR LIQUEFACTION PROCESS
WARNING: FOR EMERGENCY USE ONLY WHEN ADMINISTERED BY PROPERLY TRAINED PERSONNEL FOR OXYGEN DEFICIENCY AND RESUSCITATION. FOR ALL OTHER MEDICAL APPLICATIONS Rx ONLY. UNINTERRUPTED USE OF HIGH CONCENTRATIONS OF OXYGEN OVER A LONG DURATION, WITHOUT MONITORING ITS EFFECT ON OXYGEN CONTENT OF ARTERIAL BLOOD MAY BE HARMFUL. USE ONLY WITH PRESSURE REDUCING EQUIPMENT AND APPARATUS DESIGNED FOR OXYGEN. DO NOT ATTEMPT TO USE ON PATIENTS WHO HAVE STOPPED BREATHING, UNLESS USED IN CONJUNCTION WITH RESUSCITATIVE EQUIPMENT.
WARNING: EXTREME COLD OXIDIZING LIQUID AND GAS UNDER PRESSURE. OXYGEN VIGOROUSLY ACCELERATES COMBUSTION. COMBUSTIBLES IN CONTACT WITH LIQUID OXYGEN MAY EXPLODE ON IGNITION OR IMPACT. CAN CAUSE SEVERE FROSTBITE. OPEN VALVE SLOWLY AND CLOSE AFTER EACH USE. KEEP AWAY FROM HEAT, FLAME, OR SPARKS. KEEP OIL, GREASE, AND OTHER COMBUSTIBLES AWAY. USE ONLY WITH EQUIPMENT CLEANED FOR OXYGEN SERVICE. DO NOT STRIKE ARC OR LASER BEAM ON CYLINDER. DO NOT GET LIQUID IN EYES, ON SKIN OR CLOTHING. CONTAINER TEMPERATURE SHOULD NOT EXCEED 52 C (125 F) FOR LIQUID WITHDRAWAL, USE FACE SHIELD AND GLOVES. USE SUITABLE HAND TRUCK FOR CONTAINER MOVEMENT. DO NOT WALK ON OR ROLL EQUIPMENT OVER SPILLS. TRANSPORT, STORE AND USE ADEQUATE VENTILATION. ALWAYS SECURE CYLINDER IN UPRIGHT POSITION. USE THIS PRODUCT IN ACCORDANCE WITH ITS SPECIFIC MATERIAL SAFETY DATA SHEET (MSDS), AVAILABLE AT ATLANTA MEDICAL GAS LLC. DO NOT REMOVE THIS PRODUCT LABEL. L/334 CAS# 7782-44-7
CONTENTS
CYLINDER TYPE LITERS
160L 123048
180L 137056
200L 142858
230L 167819