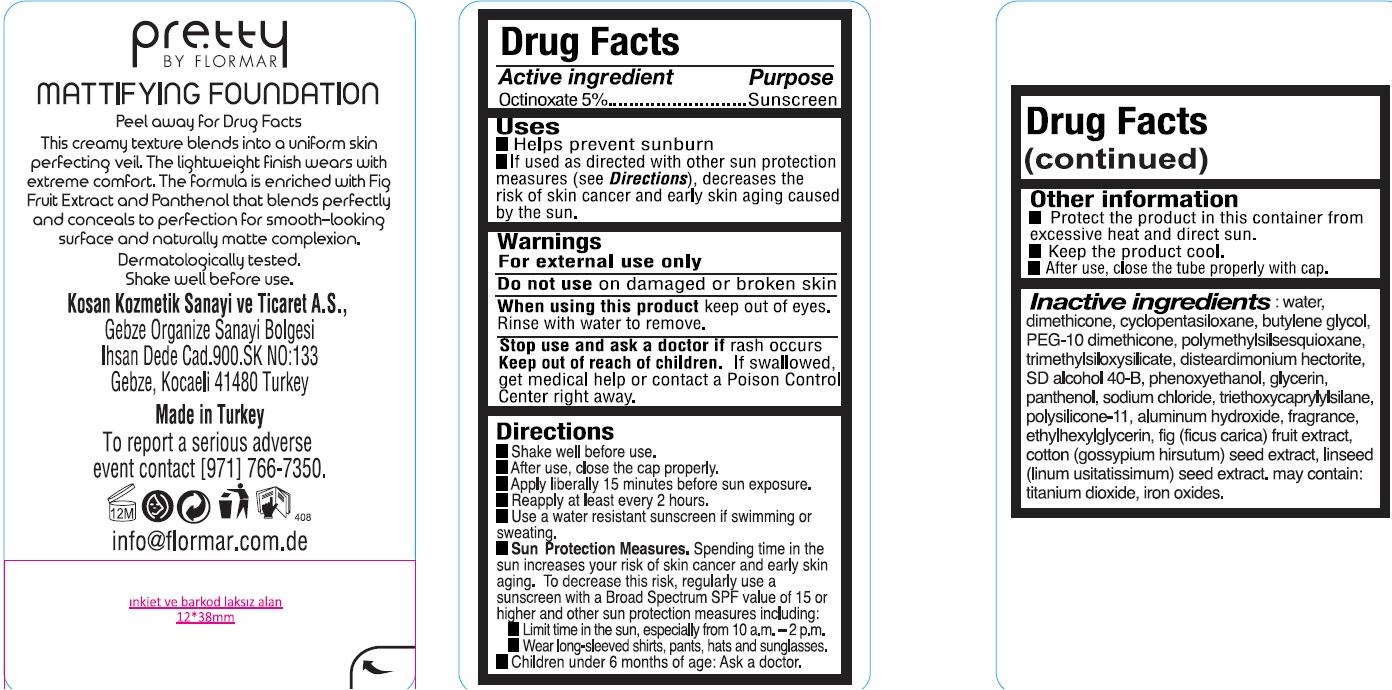
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Shake well before use.
- After use, close the cap properly.
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. -2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Children under 6 months of age: Ask a doctor.
Other information
- Protect the product in this container from excessive heat and direct sun.
- Keep the product cool.
- After use, close the tube properly with cap.
Inactive ingredients:
water, dimethicone, cyclopentasiloxane, butylene glycol, PEG-10 dimethicone, polymethylsilsesquioxane, trimethylsiloxysilicate, disteardimonium hectorite, SD alcohol 40-B, phenoxyethanol, glycerin, panthenol, sodium chloride, triethoxycaprylylsilane, polysilicone-11, aluminum hydroxide, fragrance, ethylhexylglycerin, fig (ficus carica) fruit extract, cotton (gossypium hirsutum) seed extract, linseed (linum usitatissimum) seed extract, may contain: titanium dioxide, iron oxides.