NAPHAZOLINE HYDROCHLORIDE AND PHENIRAMINE MALEATE- naphazoline hydrochloride and pheniramine maleate solution/ drops
Akorn
----------
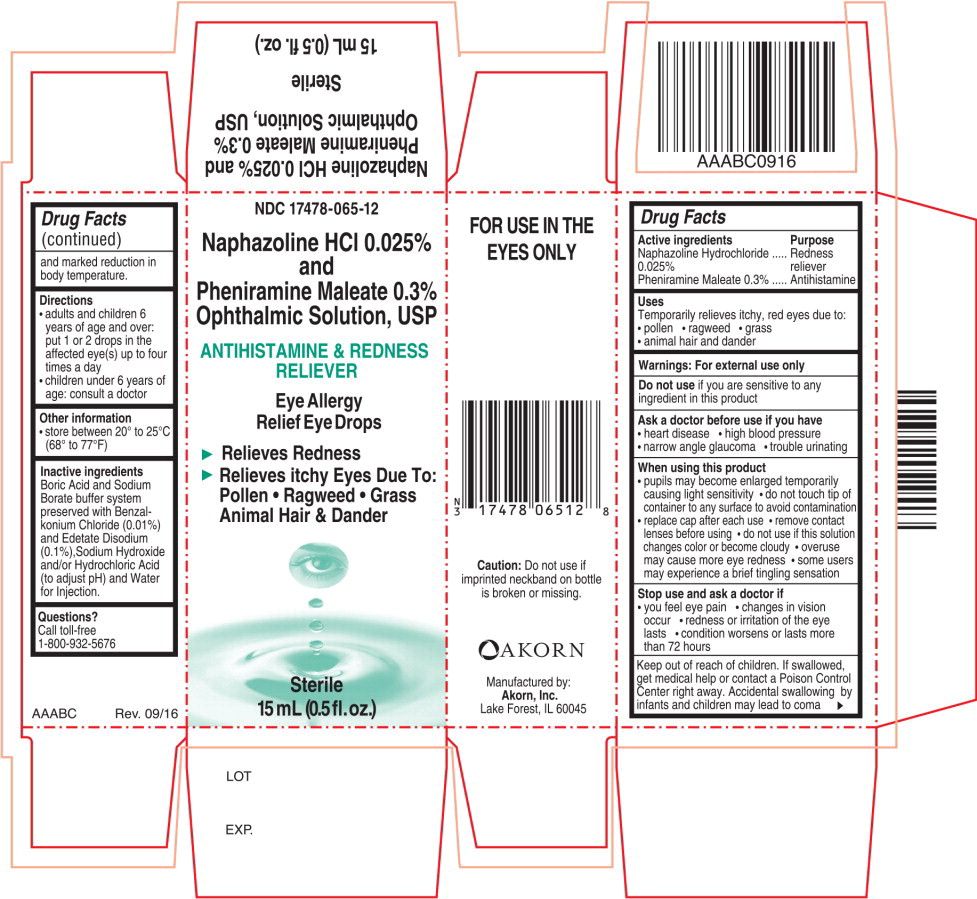
Warnings:
For external use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- narrow angle glaucoma
- trouble urinating
When using this product
- pupils may become enlarged temporarily causing light sensitivity
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- remove contact lenses before using
- do not use if this solution changes color or become cloudy
- overuse may cause more eye redness
- some users may experience a brief tingling sensation
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to four times a day
- children under 6 years of age: consult a doctor
Inactive ingredients
Boric Acid and Sodium Borate buffer system preserved with Benzalkonium Chloride (0.01%) and Edetate Disodium (0.1%), Sodium Hydroxide and/or Hydrochloric Acid (to adjust pH) and Water for Injection.
Principal Display Panel Text for Container Label:
NDC 17478-065-12
Naphazoline HCl 0.025%
and
Pheniramine Maleate 0.3%
Ophthalmic Solution, USP
ANTIHISTAMINE & REDNESS
RELIEVER
Eye Allergy
Relief Eye Drops
Sterile
15 mL (0.5 fl. oz.)
Principal Display Panel Text for Carton Label:
NDC 17478-065-12
Naphazoline HCl 0.025%
and
Pheniramine Maleate 0.3%
Ophthalmic Solution, USP
ANTIHISTAMINE & REDNESS
RELIEVER
Eye Allergy
Relief Eye Drops
► Relieves Redness
► Relieves itchy Eyes Due To:
Pollen ● Ragweed ● Grass
Animal Hair & Dander
Sterile
15 mL (0.5 fl. oz.)
| NAPHAZOLINE HYDROCHLORIDE AND PHENIRAMINE MALEATE
naphazoline hydrochloride and pheniramine maleate solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akorn (117696770) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696790 | PACK(17478-065) , LABEL(17478-065) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696832 | MANUFACTURE(17478-065) , ANALYSIS(17478-065) , STERILIZE(17478-065) | |