DESCRIPTION:
Sterile Water for Injection, USP is water for injection sterilized and packaged in single dose vials. It contains no antimicrobial agents or other preservatives. It is used as a diluent. Non-pyrogenic.
PRECAUTIONS:
Unused amount should be discarded immediately following withdrawal of any portion of vial contents. Sterile Water for Injection is not isotonic and should not be injected directly into the body.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Parenteral drug products should be inspected visually for particulate matter and discoloration, whenever solution and container permit.
HOW SUPPLIED:
Sterile Water for Injection, USP
| NDC 0517-3005-25 | 5 mL Single Dose Vial | Packaged in 25 |
| NDC 0517-3010-25 | 10 mL Single Dose Vial | Packaged in 25 |
| NDC 0517-3020-25 | 20 mL Single Dose Vial | Packaged in 25 |
| NDC 0517-3050-25 | 50 mL Single Dose Vial | Packaged in 25 |
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
IN3005
Rev. 1/09
PRINCIPAL DISPLAY PANEL - 5 mL
Contain
NDC 0517-3005-01
STERILE WATER
FOR INJECTION, USP
5 mL SINGLE DOSE VIAL
FOR DRUG DILUENT USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

Carton
STERILE WATER
FOR INJECTION, USP
NDC 0517-3005-25
25 x 5 mL
SINGLE DOSE VIALS
FOR DRUG DILUENT USE
Rx Only
No antimicrobial or other substance has been added. For use as a vehicle, solvent, or diluent for substances to be administered parenterally. Must be made approximately isotonic before intravenous use. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 11/05

PRINCIPAL DISPLAY PANEL - 10 mL
Container
NDC 0517-3010-01
STERILE WATER
FOR INJECTION, USP
10 mL SINGLE DOSE VIAL
FOR DRUG DILUENT USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

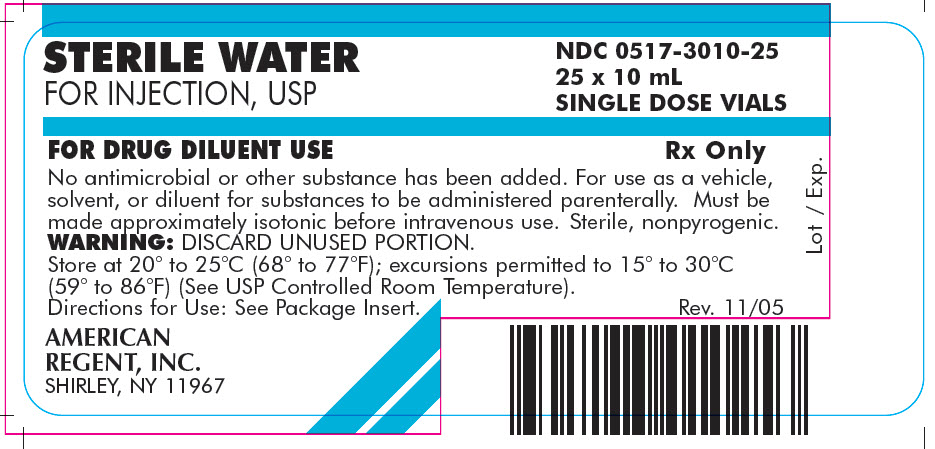
Carton
STERILE WATER
FOR INJECTION, USP
NDC 0517-3010-25
25 x 10 mL
SINGLE DOSE VIALS
FOR DRUG DILUENT USE
Rx Only
No antimicrobial or other substance has been added. For use as a vehicle, solvent, or diluent for substances to be administered parenterally. Must be made approximately isotonic before intravenous use. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 11/05
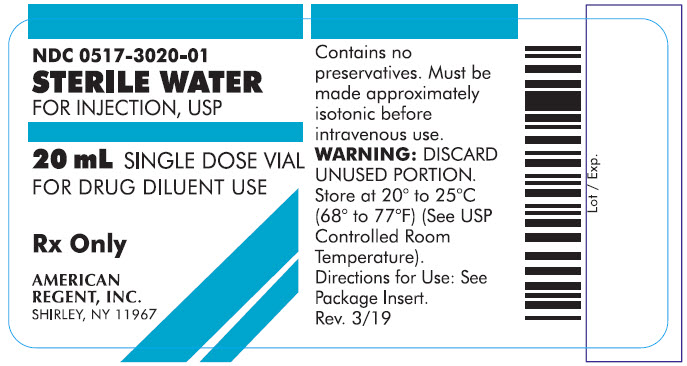
PRINCIPAL DISPLAY PANEL - 20 mL
Container
NDC 0517-3020-01
STERILE WATER
FOR INJECTION, USP
20 mL SINGLE DOSE VIAL
FOR DRUG DILUENT USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
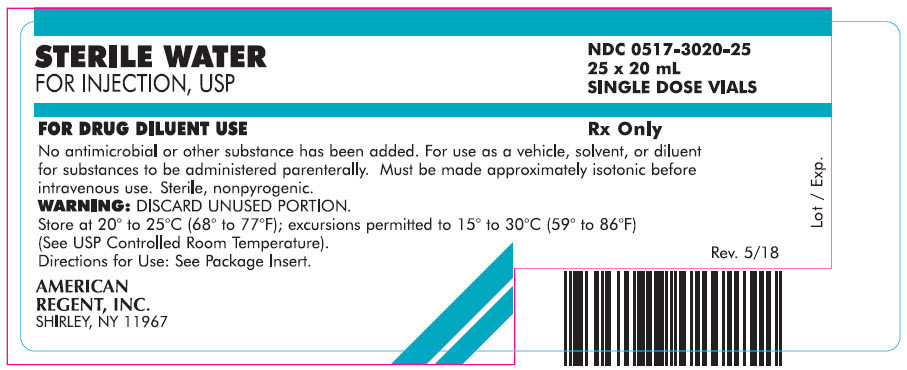
Carton
STERILE WATER
FOR INJECTION, USP
NDC 0517-3020-25
25 x 20 mL
SINGLE DOSE VIALS
FOR DRUG DILUENT USE
Rx Only
No antimicrobial or other substance has been added. For use as a vehicle, solvent, or diluent for substances to be administered parenterally. Must be made approximately isotonic before intravenous use. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 5/18