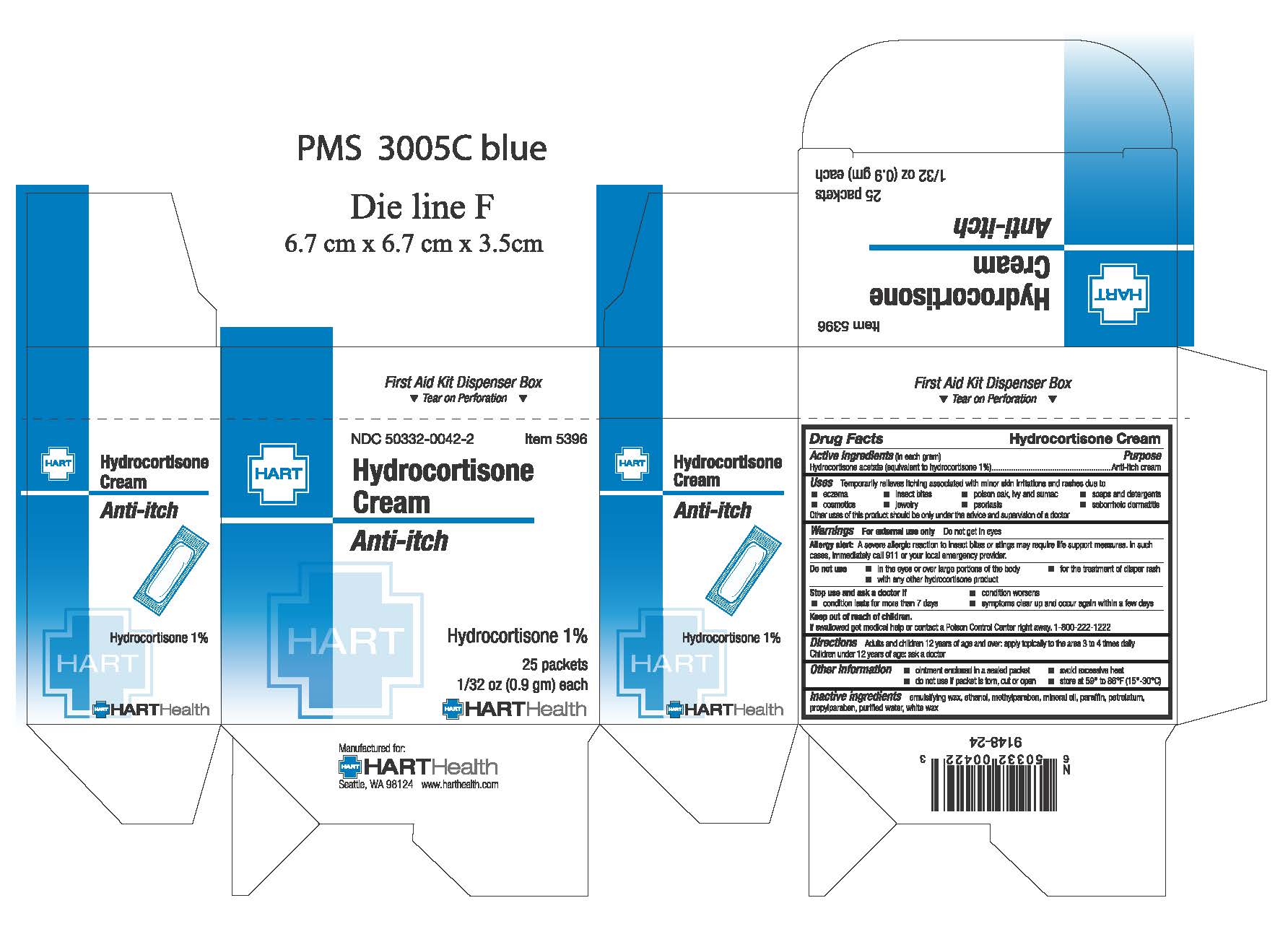
Warnings: For external use only. Do not get in eyes
Allergy alert: A severe allergic reaction to insect bites or stings may require life support measures. In such cases, immediately clal 911 or your local emergency provider.
Do not use:
- in the eyes or over large portions of the body
- for the treatment of diaper rash
- with any other hydrocortisone product
Stop use and ask a doctor if:
- condition worsens
- condition lasts for more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center right away. 1-800-222-1222
Directions:
Adults and children 12 years of age and over: apply topically to the area 3 to 4 times daily.
Children under 12 years of age: do not use, ask a doctor