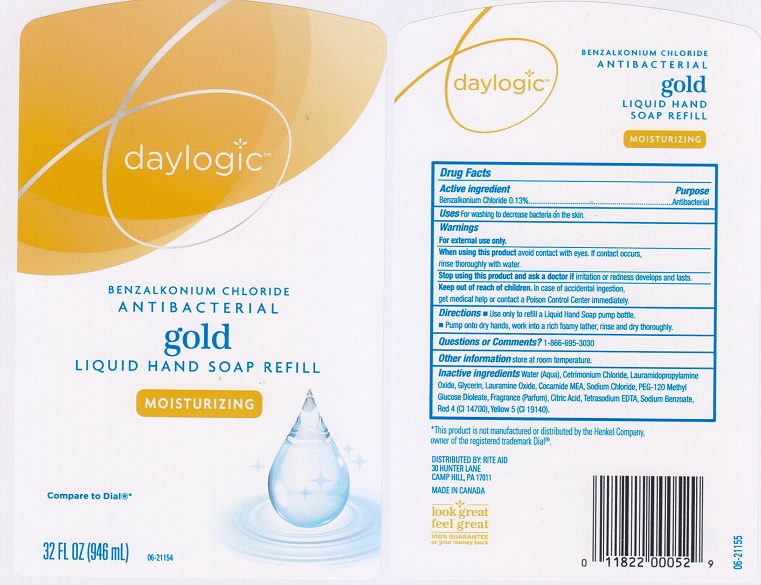
DAYLOGIC ANTIBACTERIAL GOLD REFILL- benzalkonium chloride liquid
Rite Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Benzalkonium chloride 0.13%
Uses
for washing to decrease bacteria on the skin.
Warnings
For external use only
When using this product
avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Stop using this product and ask a doctor if
irritation or redness develops and lasts.
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Directions
- use only to refill a Liquid Hand Soap pump bottle.
- Pump onto dry hands, work into a rich foamy lather, rinse and dry thoroughly.
Questions or Comments?
1-866-695-3030
Other information
store at room temperature
Inactive ingredients
Water (Aqua), Cetrimonium Chloride, Lauramidopropylamine Oxide, Glycerin, Lauramine Oxide, Cocamide MEA, Sodium Chloride, PEG-120 Methyl Glucose Dioleate, Fragrance (Parfum), Citric Acid, Tetrasodium EDTA, Sodium Benzoate, Red 4 (CI 14700), Yellow 5 (CI 19140).
Label Copy