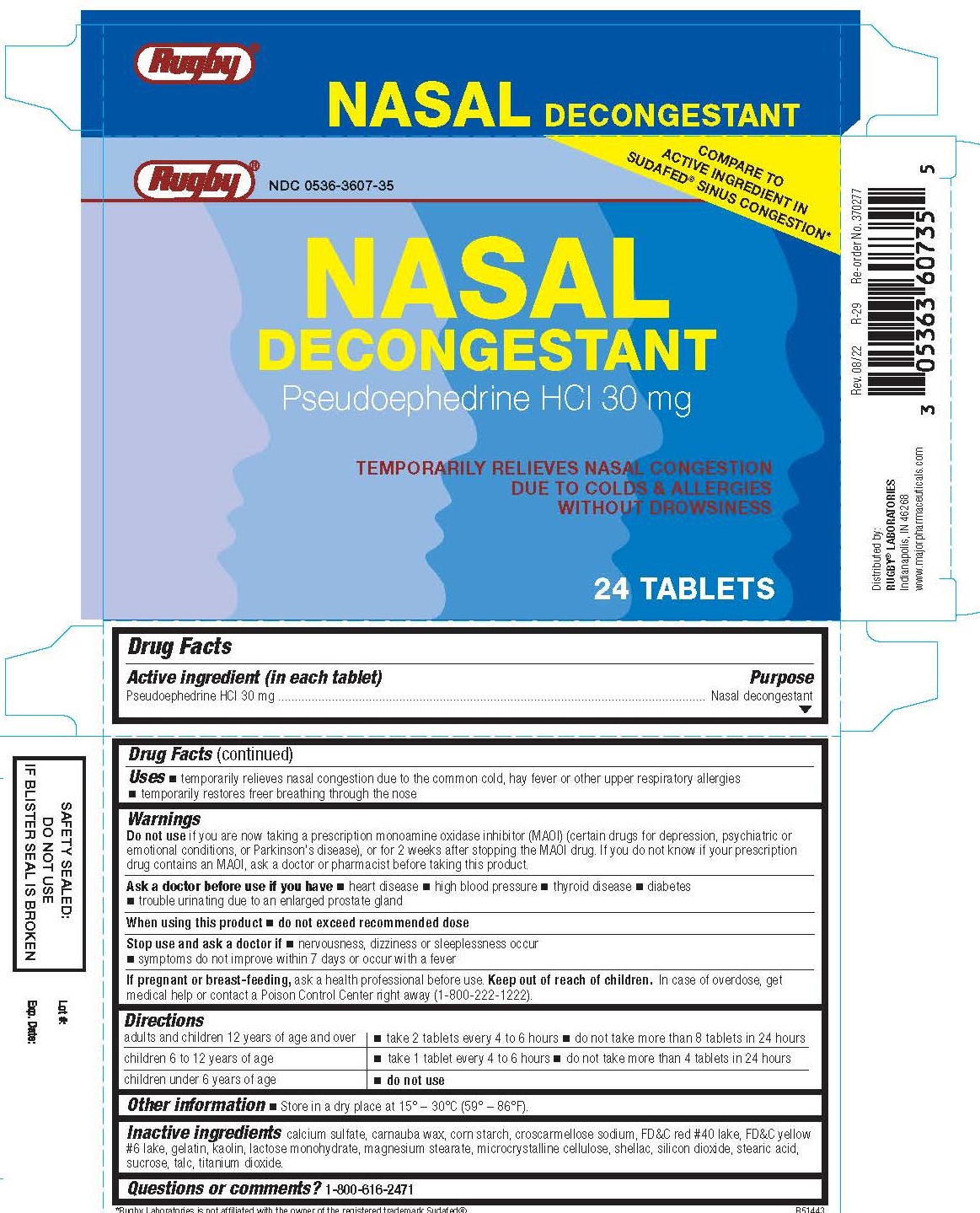
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains and MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Directions
| adults and children 12 years and older | take 2 tablets every 4 to 6 hours; do not take more than 8 tablets in 24 hours |
| children ages 6 to 12 years | take 1 tablet every 4 to 6 hours; do not take more than 4 tablets in 24 hours |
| children under 6 years | do not use |
Inactive ingredients
calcium sulfate, carnauba wax, corn starch, croscarmellose sodium, FD&C red #40 lake, FD&C yellow #6 lake, gelatin, kaolin, lactose monohydrate, magnesium stearate, microcrystalline cellulose, shellac, silicon dioxide, stearic acid, sucrose, talc, titanium dioxide.