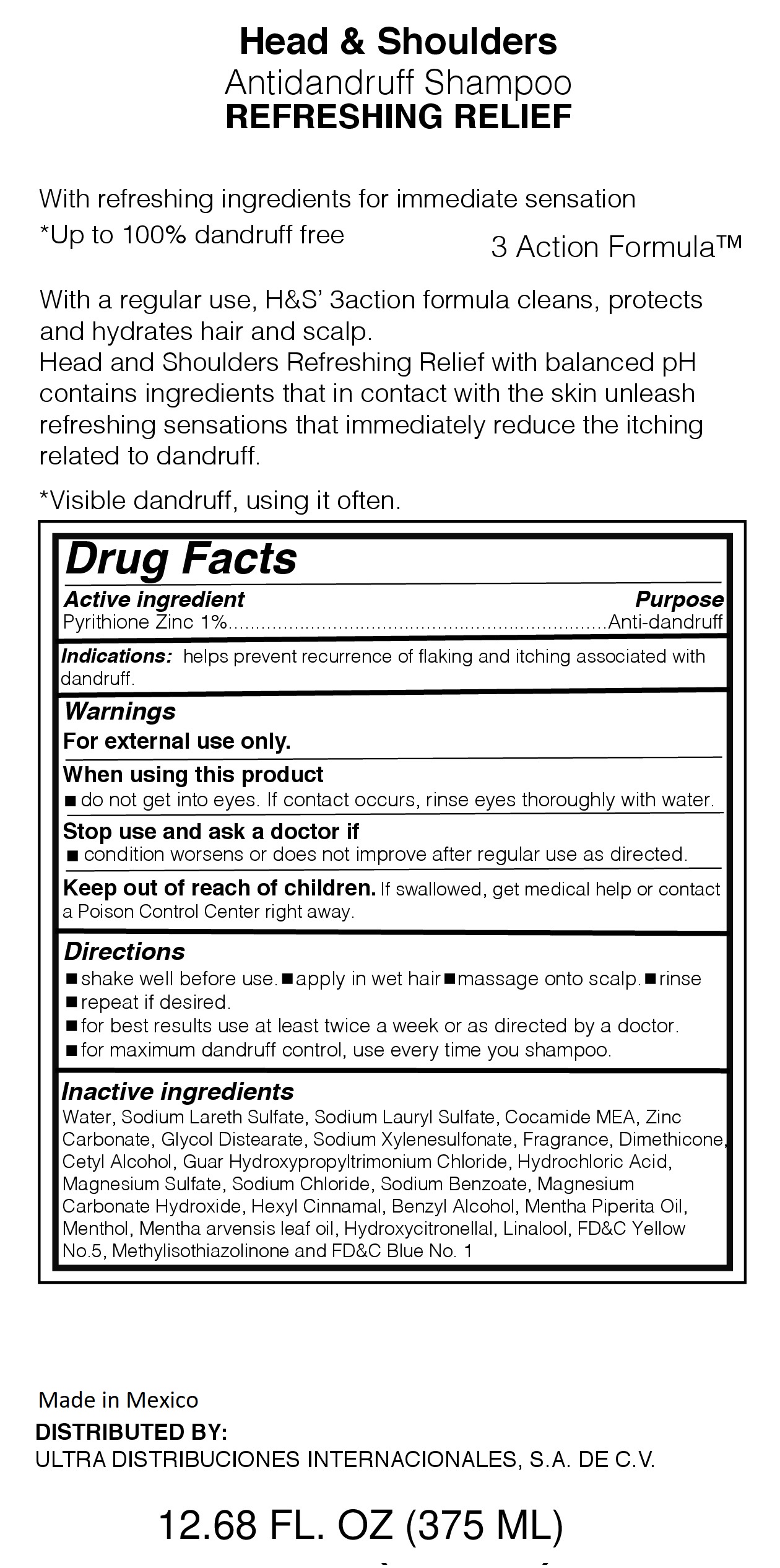
Purpose
With refreshing ingredients for immediate sensation
*up to 100% dandruff free
With Regular use, H&S 3action formula cleans, protects and hydrates hair and scalf.
Head and Shoulders Refreshing Relief with balanced PH contains ingredients that in contact with the skin unleash refreshing sensations that immediate reduce the itching related to dandruff
*Visible dandruff, using it often.
Active Ingredient
Active Ingredient................................................................................Purpose
Pyrithione Zinc 1%.....................................................................Anti-dandruff
Keep our of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
-shake well before use - apply in wet hair - massage into scalp - rinse
-repeat as desired
-for best results use at least twice a week or as directed by a doctor
-for maximum dandruff control, use every time you shampoo
Inactive Ingredients
Water, Sodium Laureth Sulfate,Sodium Lauryl Sulfate, Cocamide MEA, Zinc Carbonate,
Glycol distearate,sodium xylenesulfonate, Fragrance, Dimethicone, Cetyl Alcohol, Guar Hydroxypropyltrimonium Chloride,
Hydrochloric Acid, Magnesium Sulfate, Sodium Chloride, Sodium Benzoate, Magnesium Carbonate Hydroxide,
Hexyl Cinnamal, Benzyl Alcohol, Mentha Piperita Oil, Menthol, Mentha arvensis leaf oil, Hydroxycitronellal, Linalool,
FD&C Yellow No. 5, Methylisothiaolinone, FD&C Blue No. 1