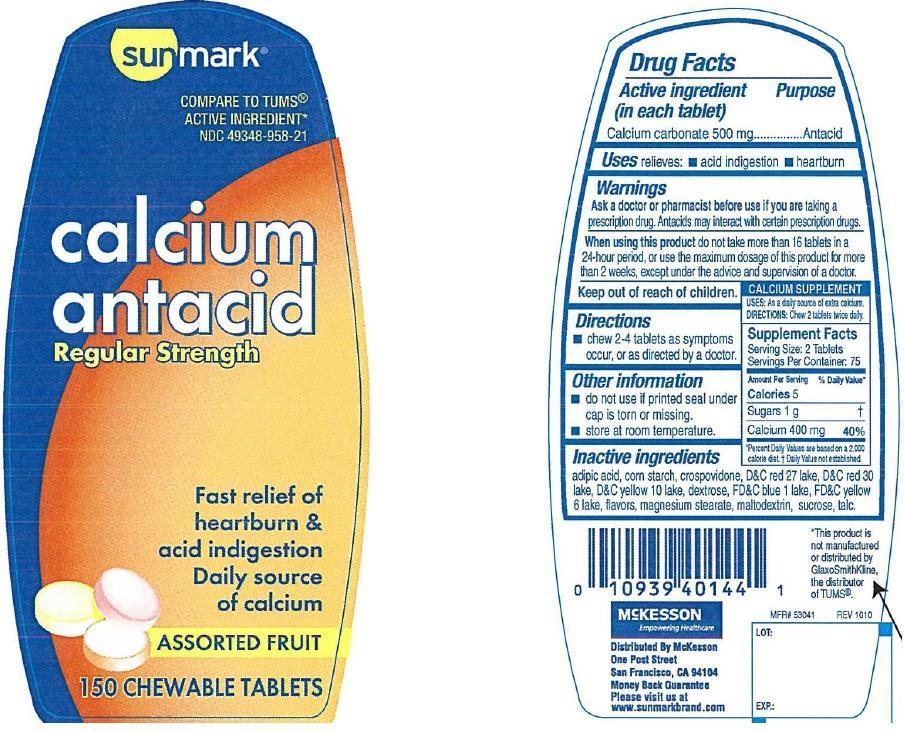
SUNMARK CALCIUM ANTACID- calcium carbonate tablet, chewable
McKesson
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each tablet)
Calcium carbonate 500 mg
Uses
relieves
- acid indigestion
- heartburn
Warnings
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Antacids may interact with certain prescriptiom drugs.
When using this product
do not take more than 16 tablets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
Keep out of reach of children
Directions
- chew 2-4 tablets as symptoms occur, or as directed by a doctor
Other information
- do not use if seal under cap is torn or missing
- store at room temperature
Inactive ingredients
adipic acid, corn starch, crospovidone, D and C red 27 lake, D and C red 30 lake, D and C Yellow 10 lake, dextrose, FD and C blue 1 lake, FD and C yellow 6 lake, flavors, magnesium stearate, maltodextrin, sucrose, talc
Principal Display Panel
Sunmark
compare to Tums active ingredient
calcium antacid
regular strength
fast relief of heartburn and acid indigestion
daily source of calcium
assorted fruit flavors
150 chewable tablets