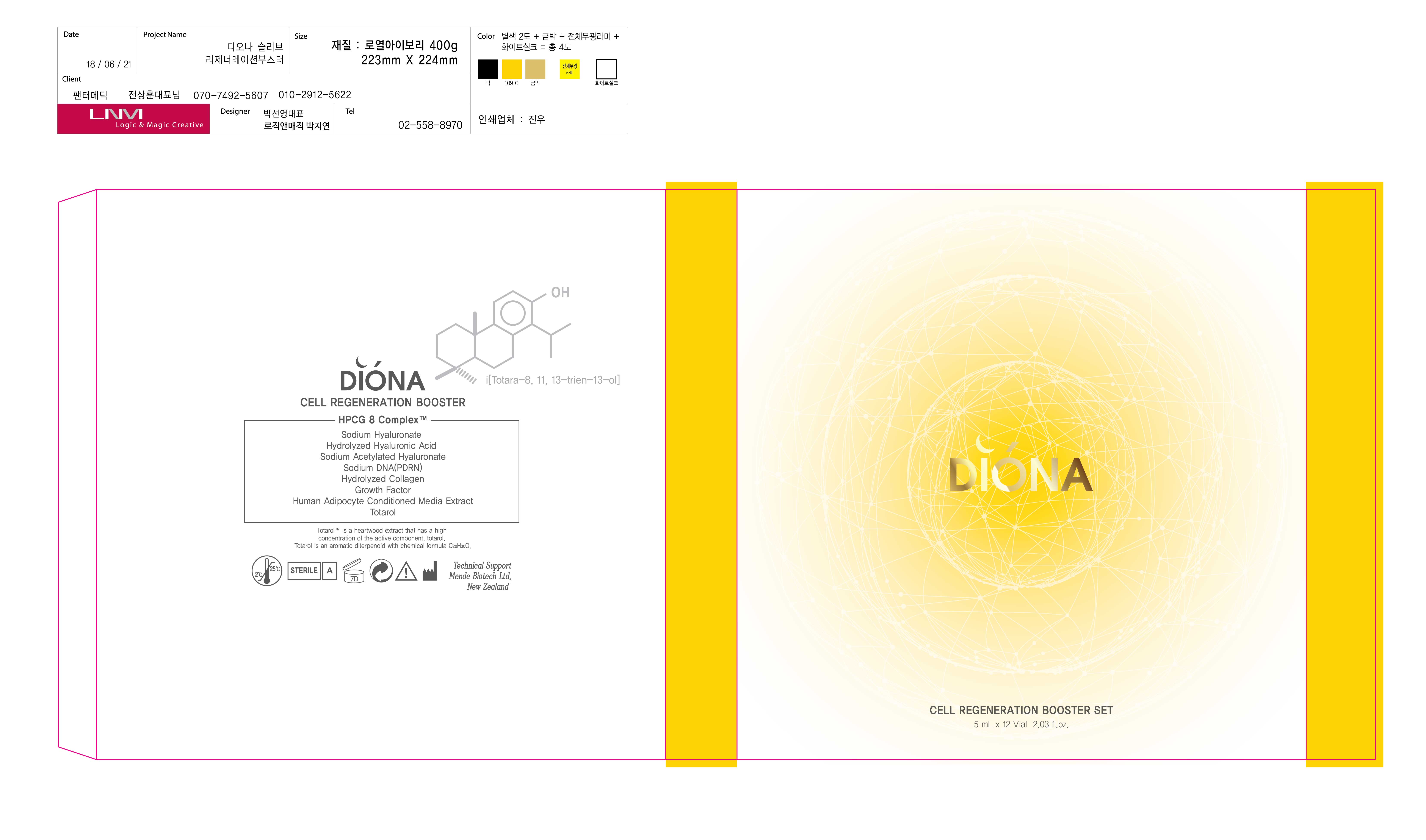
DIONA CELL REGENERATION BOOSTER- glycerin liquid
VAATZ CO., LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
WATER, 1,2-HEXANEDIOL, ADENOSINE, BUTYLENE GLYCOL, ETC.
keep out of reach of the children
Apply an appropriate amount to skin and absorb it.
1) If following symptoms occur after product use, stop using immediately and consult with a specialist: red spots, swelling, itchiness, and other skin irritations. 2) Avoid contact with eyes and open wounds. 3) Keep out of reach of children. 4) Do not store at high/low temperature. 5) Avoid direct sunlight.
VAATZ CO., LTD.