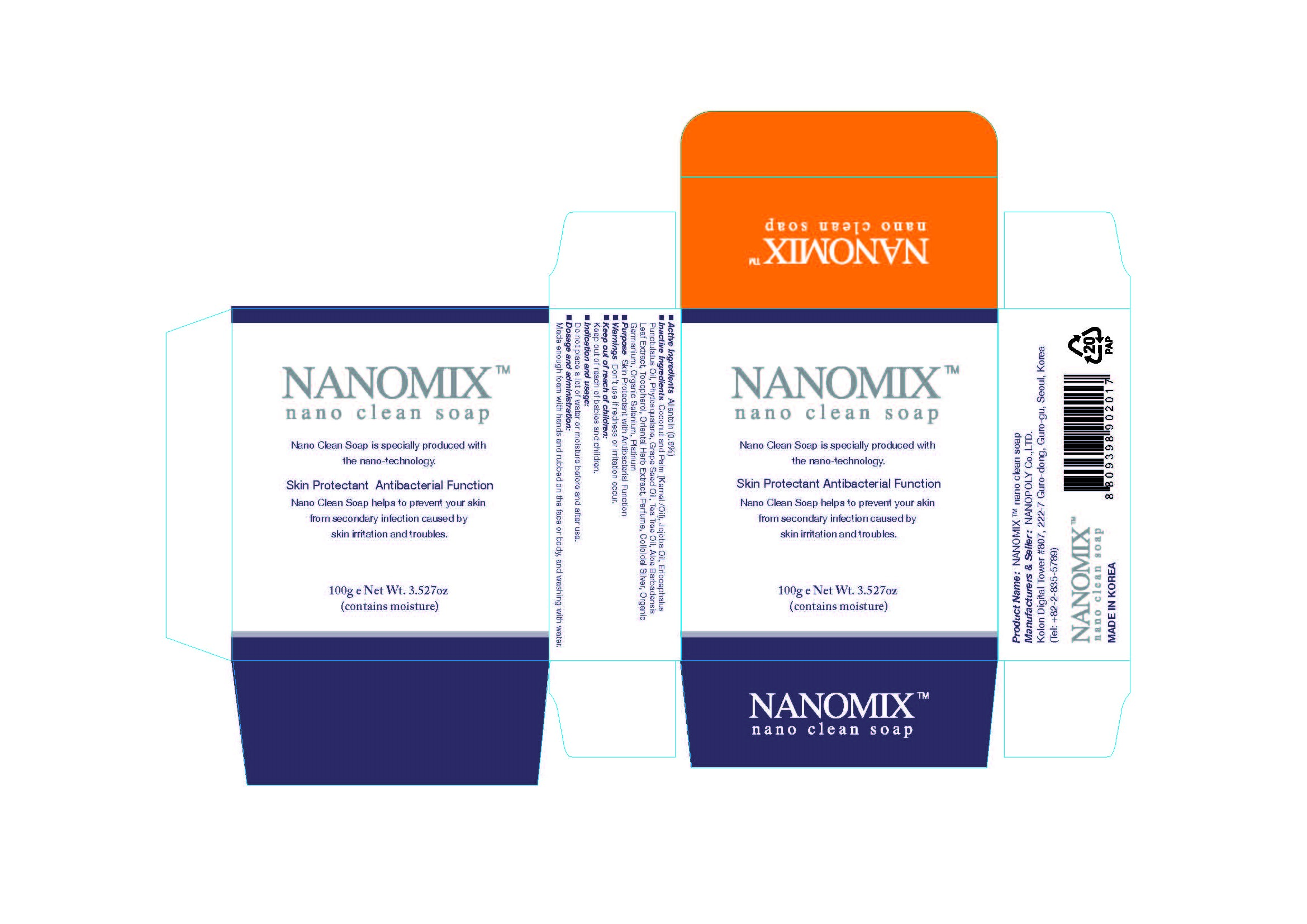
NANOMIX NANO CLEAN SKIN PROTECTANT ANTIBACTERIAL FUNCTION- allantoin soap
NANOPOLY CO., LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
Active Ingredients: Allantoin 0.6%
INACTIVE INGREDIENT
Inactive Ingredients:
Coconut and Palm (Kernel /Oil), Jojoba Oil, Eriocephalus Punctulatus Oil, Phytosqualane, Grape Seed Oil, Tea Tree Oil, Aloe Barbadensis Leaf Extract, Tocopherol, Oriental Herb Extract, Perfume, Colloidal Silver, Organic Germanium, Organic Selenium, Platinum
PURPOSE
Purpose: Skin Protectant with Antibacterial Function
WARNINGS
Warnings:
Don't use if redness or irritation occur.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children:
Keep out of reach of babies and children.
INDICATIONS AND USAGE
Indication and usage:
Do not place a lot of water or moisture before and after use.
DOSAGE AND ADMINISTRATION
Dosage and administration:
Made enough foam with hands and rubbed on the face or body, and washing with water.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NANOPOLY CO., LTD.
