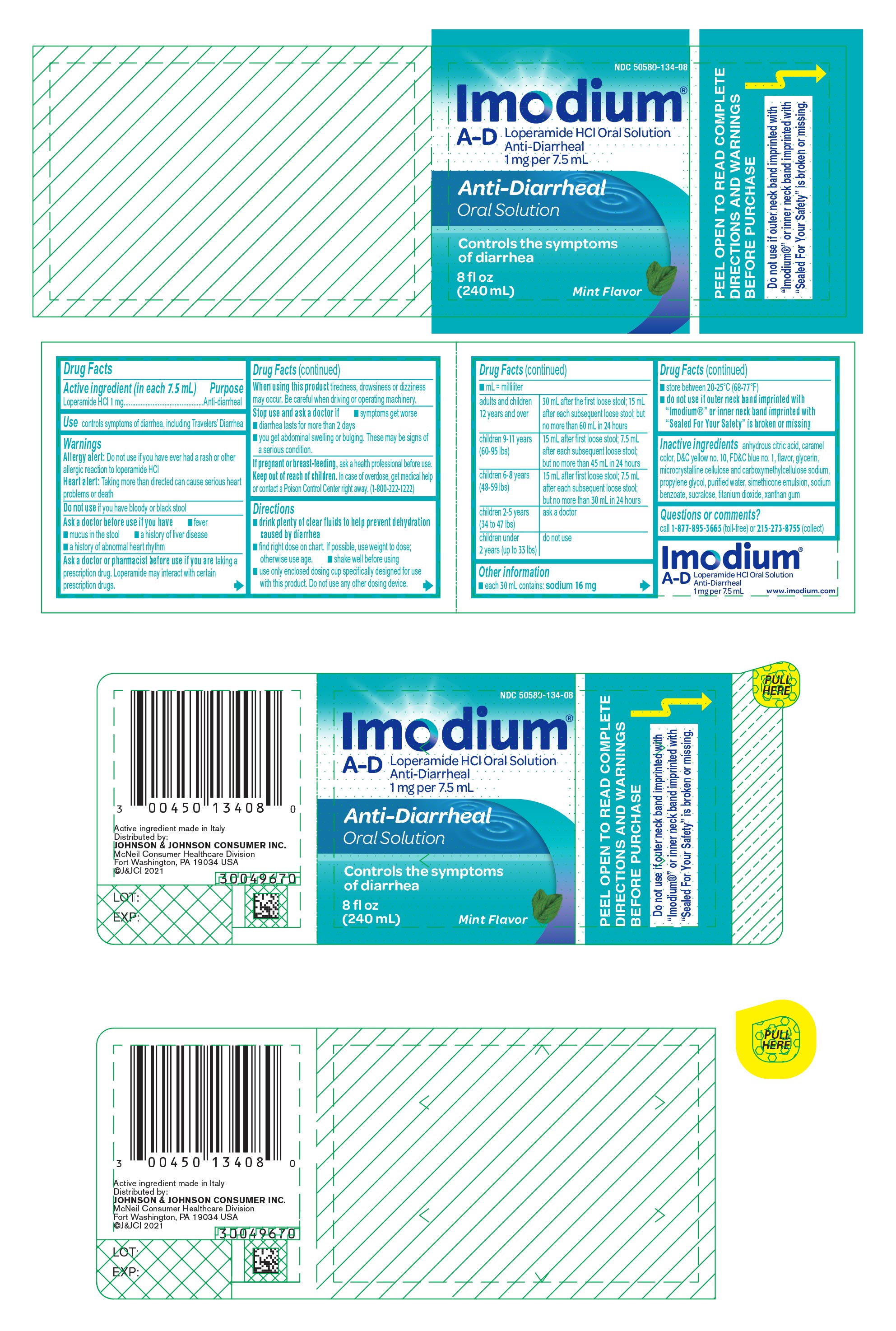
Warnings
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise use age.
- shake well before using
- use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- mL = milliliter
| adults and children 12 years and over | 30 mL after the first loose stool; 15 mL after each subsequent loose stool; but no more than 60 mL in 24 hours |
| children 9-11 years (60-95 lbs) | 15 mL after first loose stool; 7.5 mL after each subsequent loose stool; but no more than 45 mL in 24 hours |
| children 6-8 years (48-59 lbs) | 15 mL after first loose stool; 7.5 mL after each subsequent loose stool; but no more than 30 mL in 24 hours |
| children 2-5 years (34 to 47 lbs) | ask a doctor |
| children under 2 years (up to 33 lbs) | do not use |
Other information
- each 30 mL contains: sodium 16 mg
- store between 20-25°C (68-77°F)
- do not use if outer neck band imprinted with "Imodium®" or inner neck band imprinted with "Sealed For Your Safety" is broken or missing