FIRST AID AND BURN- benzalkonium chloride, lidocaine hydrochloride cream
Acme United Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
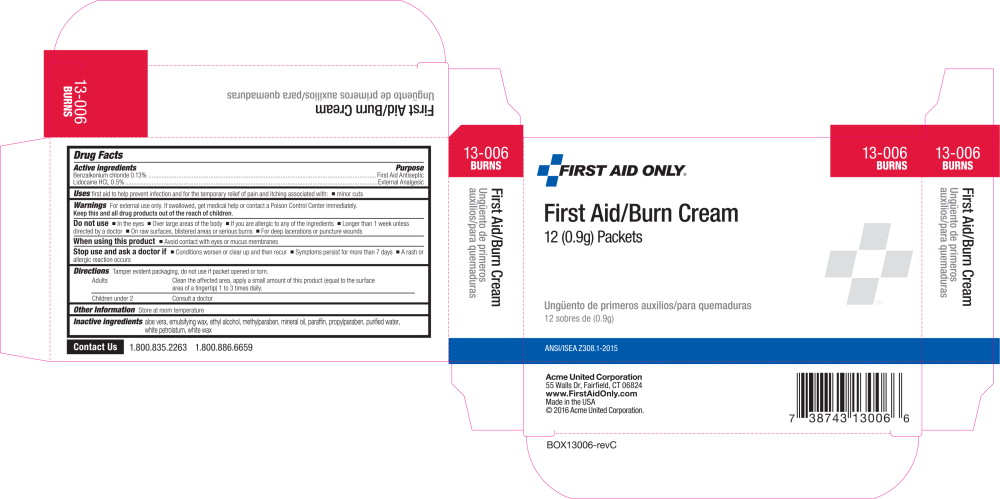
Active Ingredients
Benzalkonium Chloride 0.13%
Lidocaine HCl 0.5%
Purpose
First Aid Antiseptic
External analgesic
Uses
first aid to help prevent infection and for the temporary relief of itching associated with
Warnings
For external use only
Do not use
- in the eyes or apply over large areas of the body
- longer than 1 week unless directed by a doctor
- in large quantities, particularly over raw surfaces or blistered areas
Ask a doctor before use if you have deep or puncture wounds, animal bites, or serious burns
When using this product avoid contact with eyes
Stop use and ask a doctor if
- condition worsens or persists for more than 7 days
- clears up and occurs again within a few days
Keep out of reach of children If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product to the area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
Other information
- store at room temperature
- do not use if packet is opened or torn
Inactive ingredients
aloe vera, emulsifying wax, ethyl alcohol, methylparaben, mineral oil, paraffin, propylparaben, purified water, white petrolatum, white wax
Questions? 1-800-835-2263
REORDER
www.PhysiciansCareFirstAid.com
1 800 835 2263
Principal Display Panel - Packet Label
FIRST AID ONLY®
Burn Cream
NET WT 1/32 oz (0.9g)
© 2015 Acme United Corporation.
Faifield, CT 06824
1.800.835.2263
www.FirstAidOnly.com
NDC 0924-5701-01
Principal Display Panel - Packet Label
FIRST AID ONLY®
13-006
BURNS
First Aid/Burn Cream
12 (0.9g) Packets
Ungüento de primeros auxilios/para quemaduras
12 sobres de (0.9g)
NDC 0924-5701-10