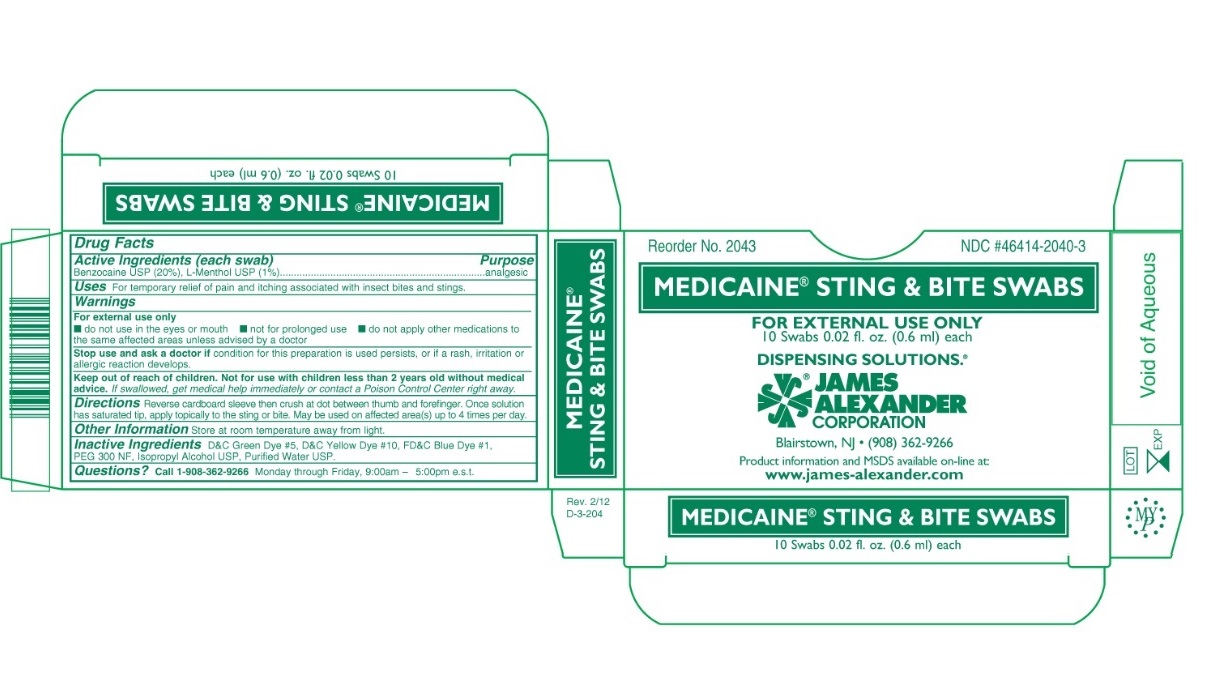
Warnings
For external use only
- do not use in the eyes or mouth
- not for prolonged use
- do not apply other medications to the same affected areas unless advised by a doctor
Stop use and ask a doctor if condition for this preparation is used persists, or if a rash or irritation or allergic reaction develops.
Keep out of reach of children. Not for use with children less than 2 years old without medical advice. If swallowed, get medical help immediately or contact a Poison Control Center right away.
Directions
Reverse cardboard sleeve then crush at dot between thumb and forefinger. Once solution has saturated tip, apply topically to the sting or bite. May be used on affected area(s) up to 4 times per day.
Inactive Ingredients
D&C Green Dye #5, D&C Yellow Dye #10, FD&C Blue Dye #1, PEG 300 NF, Isopropyl Alcohol USP, Purified Water USP.