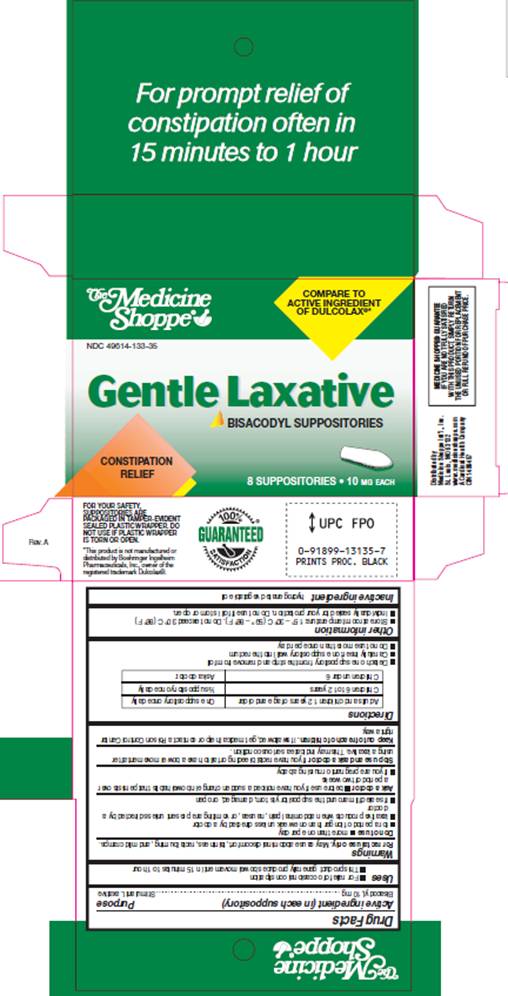
USES
- For relief of occasional constipation
- This product generally produces bowel movement in 15 minutes to 1 hour
WARNINGS
For rectal use only. May cause abdominal discomfort, faintness, rectal burning, and mild cramps.
Do not use
- more than one per day
- for a period of longer than one week unless directed by a doctor
- laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor
- if seal under product lid is torn, damaged, or open
Ask a doctor before use
- if you have noticed a sudden change in bowel habits that persist over a period of two weeks
- if you are pregnant or nursing a baby
DIRECTIONS
| Adults and children 12 years of age and older | One suppository once daily |
| Children 6 to 12 years | 1/2 suppository once daily |
| Children under 6 | Ask a doctor |
- Detach one suppository from the strip and remove from foil
- Carefully insert one suppository well into the rectum
- Do not use more than once per day
OTHER INFORMATION
- Store at room temperature: 15°- 30° C (59° - 86° F). Do not exceed 30° C (86° F).
- Individually sealed for your protection. Do not use if foil is torn or open.
PACKAGE INFORMATION
The Medicine Shoppe®
COMPARE TO ACTIVE INGREDIENT OF DULCOLAX®*
NDC 49614-133-35
Gentle Laxative
BISACODYL SUPPOSITORIES
CONSTIPATION RELIEF
8 SUPPOSITORIES 10 MG EACH
FOR YOUR SAFETY, SUPPOSITORIES ARE PACKAGED IN PAMPER-EVIDENT SEALED PLASTIC WRAPPER. DO NOT USE IF PLASTIC WRAPPER IS TORN OR OPEN.
*This product is not manufactured or distributed by Boehringer Ingelhem
Pharmaceuticals, Inc., owner of the registered trademark Dulcolax®.
Another Quality Product
Distributed by Medicine Shoppe Int'l., Inc.
St. Louis, MO 63132
www.medicineshoppe.com
A Cardinal Health Company