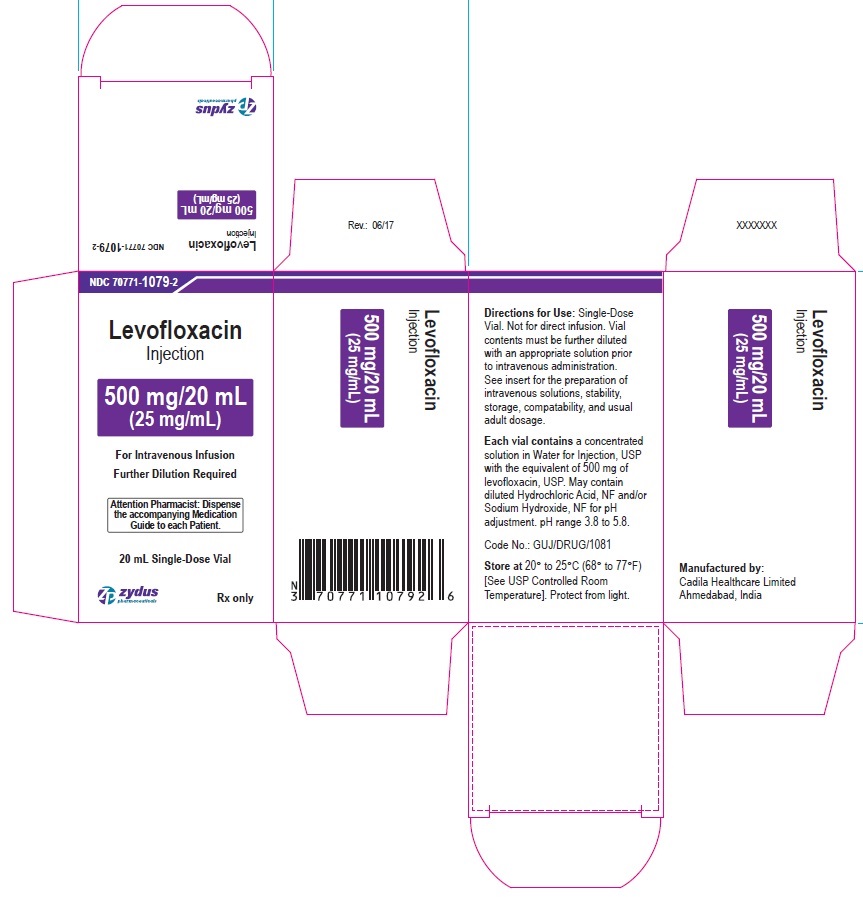
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 MG/20 ML CONTAINER LABEL
NDC 70771-1079-2
Levofloxacin
Injection
500 mg/20 mL
(25 mg/mL)
For Intravenous Infusion
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
20 mL Single-Dose Vial
Rx only
zydus pharmaceuticals

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 MG/20 ML CARTON LABEL
NDC 70771-1079-2
Levofloxacin
Injection
500 mg/20 mL
(25 mg/mL)
For Intravenous Infusion
Further Dilution Required
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
20 mL Single-Dose Vial
Rx only
zydus pharmaceuticals
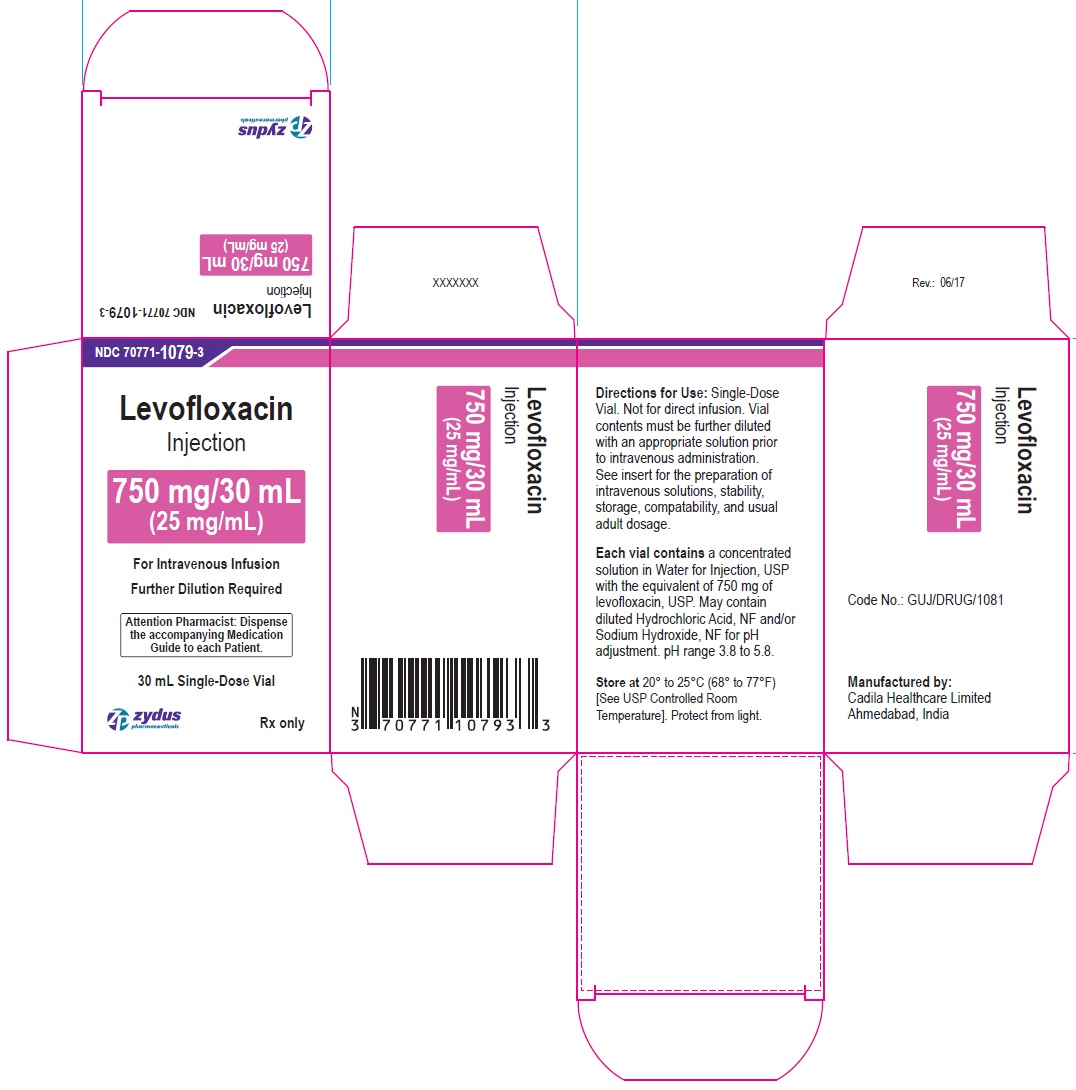
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 750 MG/30 ML CONTAINER LABEL
NDC 70771-1079-3
Levofloxacin
Injection
750 mg/30 mL
(25 mg/mL)
For Intravenous Infusion
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
30 mL Single-Dose Vial
Rx only
zydus pharmaceuticals

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 750 MG/30 ML CARTON LABEL
NDC 70771-1079-3
Levofloxacin
Injection
750 mg/30 mL
(25 mg/mL)
For Intravenous Infusion
Further Dilution Required
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
30 mL Single-Dose Vial
Rx only
zydus pharmaceuticals