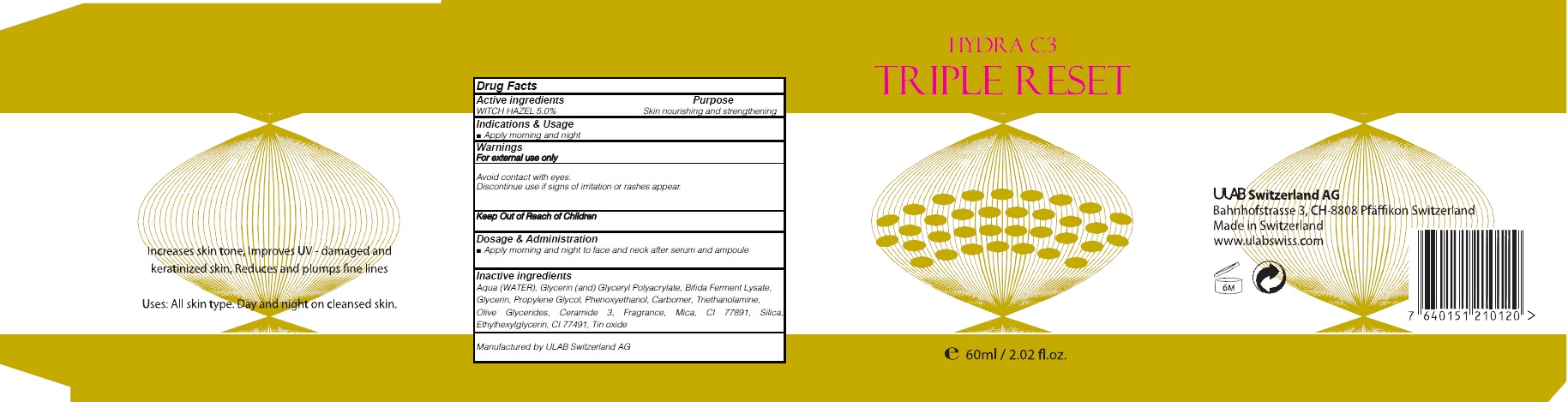
HYDRA C3 TRIPLE RESET- witch hazel cream
ULAB
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
Active ingredients: WITCH HAZEL 5.0%
INACTIVE INGREDIENT
Inactive ingredients: Aqua (WATER), Glycerin (and) Glyceryl Polyacrylate, Bifida Ferment Lysate, Glycerin, Propylene Glycol, Phenoxyethanol, Carbomer, Triethanolamine, Olive Glycerides, Ceramide 3, Fragrance, Mica, CI 77891, Silica, Ethylhexylglycerin, CI 77491, Tin oxide
PURPOSE
Purpose: Skin nourishing and strengthening
WARNINGS
Warnings: For external use only Avoid contact with eyes. Discontinue use if signs of irritation or rashes appear.
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
INDICATIONS & USAGE
Indications & Usage: Apply morning and night
DOSAGE & ADMINISTRATION
Dosage & Administration: Apply morning and night to face and neck after serum and ampoule
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL