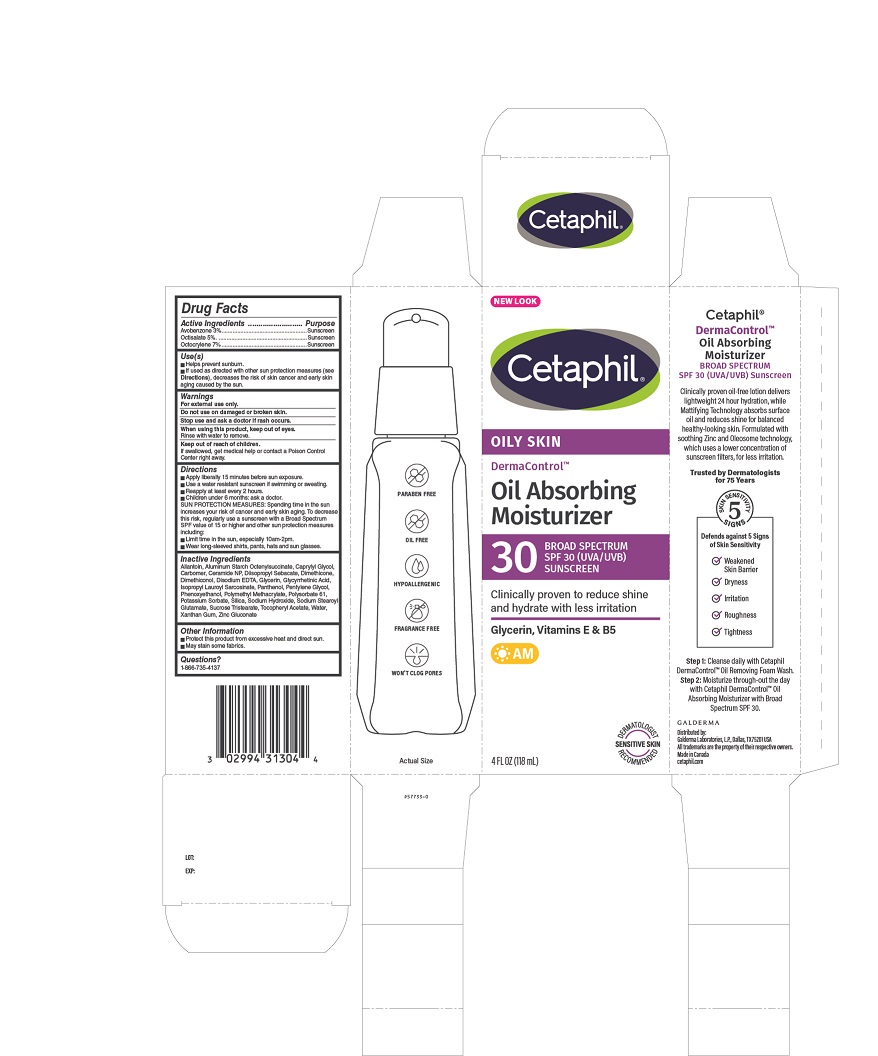
Use(s)
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
When using this product, keep out of eyes.
Rinse with water to remove.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• Apply liberally 15 minutes before sun exposure.
• Use a water resistant sunscreen if swimming or sweating.
• Reapply at least every 2 hours.
• Children under 6 months: Ask a doctor.
SUN PROTECTION MEASURES: Spending time in the sun increases your risk of cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially 10am - 2pm.
• Wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Allantoin, Aluminum Starch Octenylsuccinate, Caprylyl Glycol, Carbomer, Ceramide NP, Diisopropyl Sebacate, Dimethicone, Dimethiconol, Disodium EDTA, Glycerin, Glycyrrhetinic Acid, Isopropyl Lauroyl Sarcosinate, Panthenol, Pentylene Glycol, Phenoxyethanol, Polymethyl Methacrylate, Polysorbate 61, Potassium Sorbate, Silica, Sodium Hydroxide, Sodium Stearoyl Glutamate, Sucrose Tristearate, Tocopheryl Acetate, Water, Xanthan Gum, Zinc Gluconate
Questions?
1-866-735-4137
Distributed By:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their respective owners.
Made in Canada
cetaphil.com
PRINCIPLE DISPLAY PANEL - 4oz carton
NEW LOOK
Cetaphil®
OILY SKIN
DermaControlTM
Oil Absorbing
Moisturizer
30 BROAD SPECTRUM
SPF 30 (UVA/UVB)
SUNSCREEN
Clinically proven to reduce shine
and hydrate with less irritation
Glycerin, Vitamins E & B5
AM
Dermatologist Recommended
Sensitive Skin
4 FL OZ (118 mL)
P57733-0