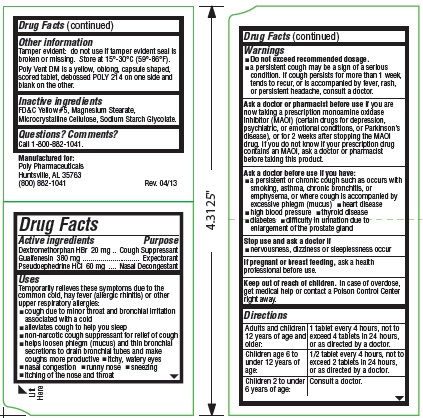
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation associated with a cold
- alleviates cough to help you sleep
- non-narcotic cough suppressant for relief of cough
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- itchy, watery eyes
- nasal congestion
- runny nose
- sneezing
- itching of the nose and throat
Warnings
-
Do not exceed recommended dosage.
- a persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
Ask a doctor or pharmacist before use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have:
- a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or where cough is accompanied by excessive phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
Directions
| Adults and children 12 years of age and over: | 1 tablet every 4 hours, not to exceed 4 tablets in 24 hours, or as directed by a doctor. |
| Children age 6 to under 12 years of age: | ½ tablet every 4 hours, not to exceed 2 tablets in 24 hours, or as directed by a doctor. |
| Children 2 to under 6 years of age: | Consult a doctor. |
Other information
Tamper evident: do not use if tamper evident seal is broken or missing. Store at 15°-30°C (59°-86°F).
Poly Vent DM is a yellow, oblong, capsule shaped, scored tablet, debossed POLY 214 on one side and
blank on the other.
Inactive ingredients
FD&C Yellow #5, Magnesium Stearate, Microcrystalline Cellulose, Sodium Starch Glycolate.