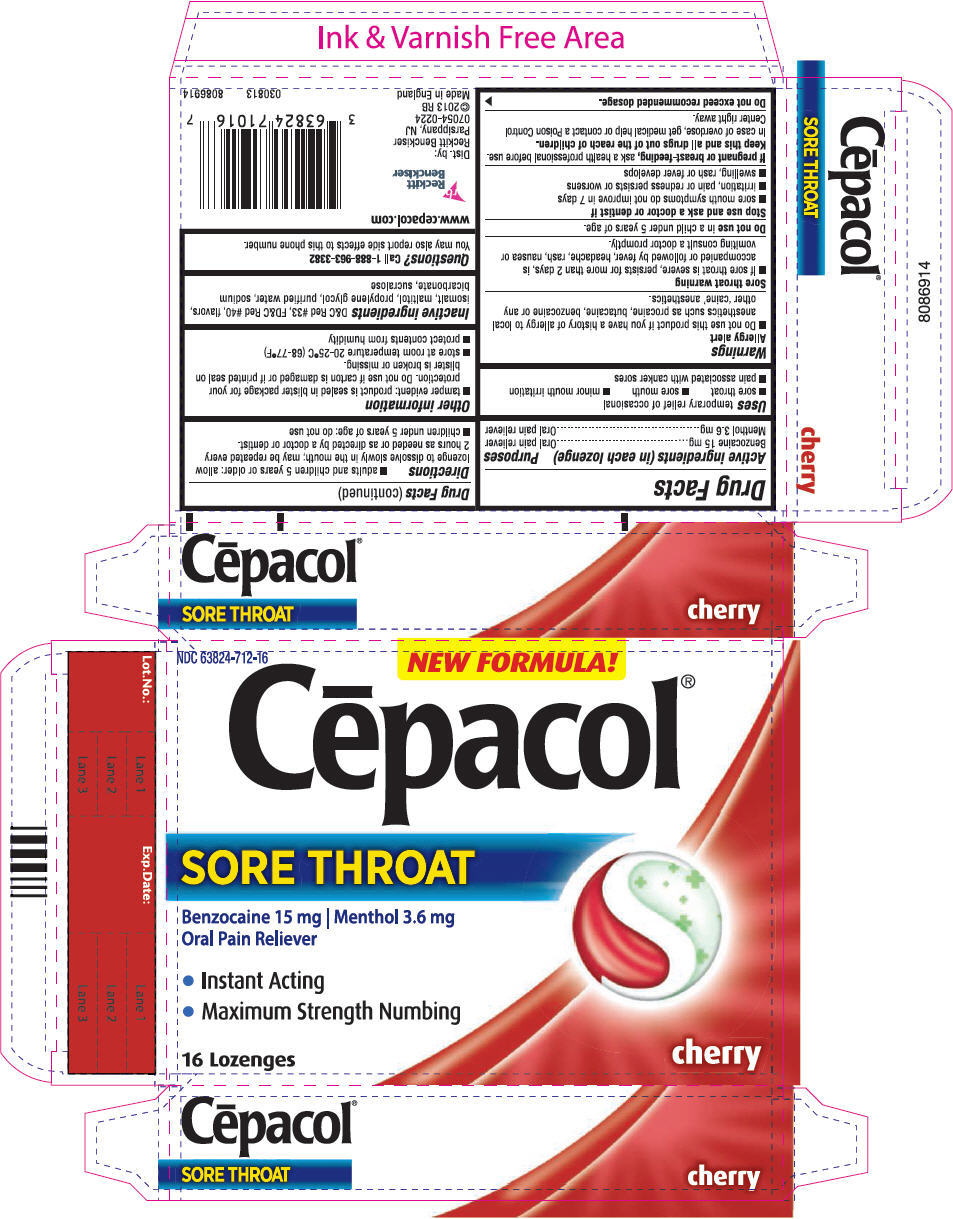
CEPACOL SORE THROAT- benzocaine and menthol lozenge
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cēpacol
®
Sore Throat
Uses
temporary relief of occasional
- sore throat
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
Allergy alert
- Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning
- If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain or redness persists or worsens
- swelling, rash or fever develops
Directions
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use
Other information
- tamper evident: product is sealed in blister package for your protection. Do not use if carton is damaged or if printed seal on blister is broken or missing.
- store at room temperature 20-25°C (68-77°F)
- protect contents from humidity
| CEPACOL
SORE THROAT
benzocaine and menthol lozenge |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |
Revised: 7/2023
Document Id: 017ee4a0-7b26-f05c-e063-6294a90a839e
Set id: 7377f536-5196-4c78-a94c-16e43d899b97
Version: 3
Effective Time: 20230727
RB Health (US) LLC