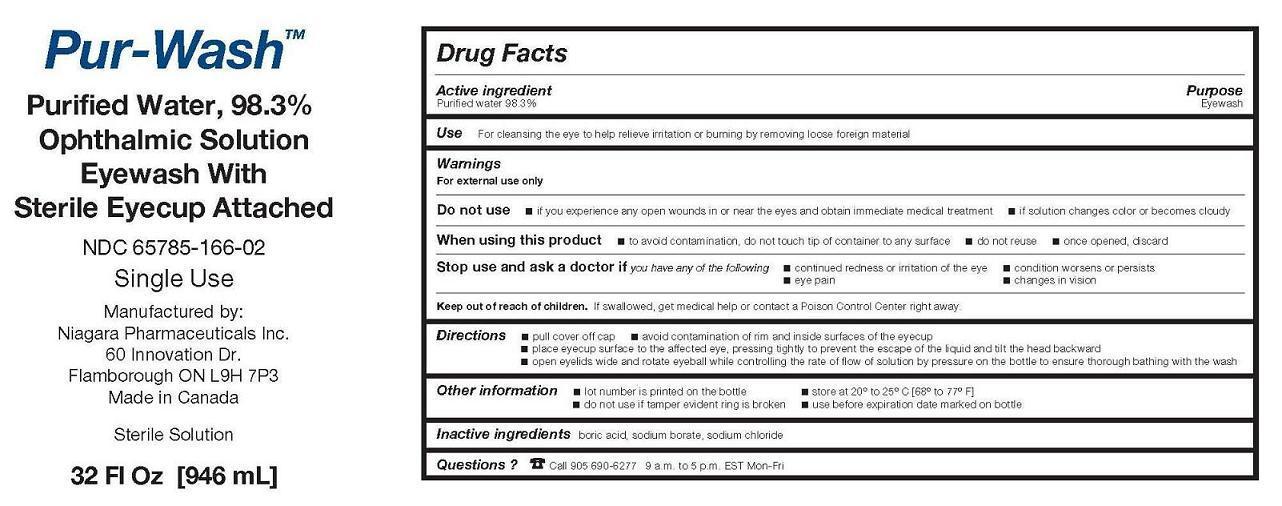
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
Directions
• pull cover off cap
• avoid contamination of rim and inside surfaces of the eyecup
• place eyecup surface to the affected eye, pressing tightly to prevent the escape of the liquid and tilt the head backward
• open eyelids wide and rotate eyeball while controlling the rate of flow of solution by pressure on the bottle to ensure thorough bathing with the wash
Other information
- lot number is printed on the bottle
- store at 20° to 25° C [68° to 77° F]
- do not use if tamper evident ring is broken
- use before expiration date marked on bottle
PRINCIPAL DISPLAY PANEL - 946 mL Bottle Label
Pur-Wash TM
Purified Water, 98.3%
Ophthalmic Solution
Eyewash With
Sterile Eyecup Attached
NDC 65785-166-02
Single Use
Manufactured by:
Niagara Pharmaceuticals Inc.
60 Innovation Dr.
Flamborough ON L9H 7P3
Made in Canada
Sterile Solution
32 Fl Oz [946 mL]