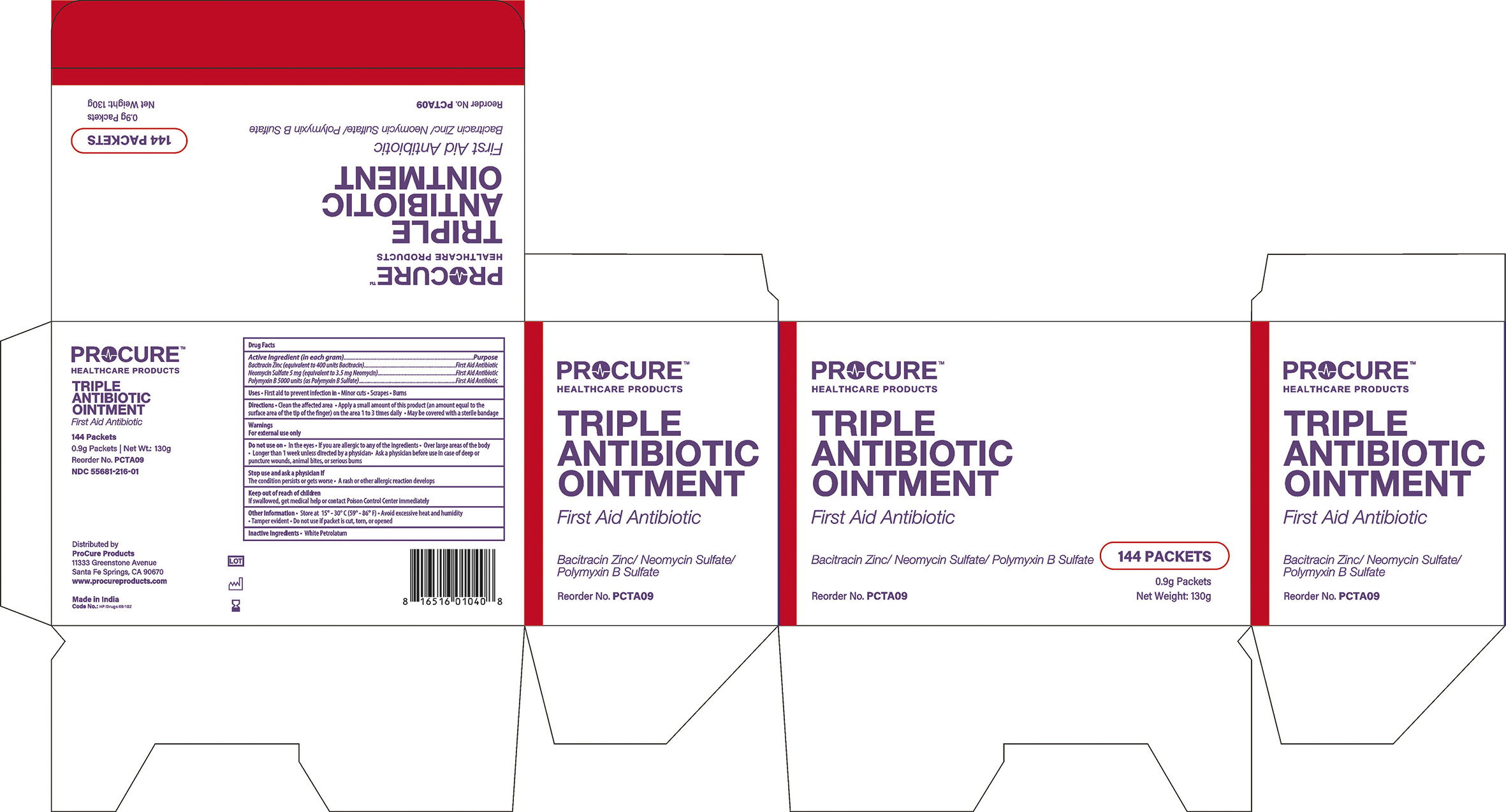
Active Ingredients(in each gram)
Bacitracin Zinc............400 units
Neomycin Sulfate 5mg (equivalent to 3.5 mg Neomycin)
Polymyxin B Sulfate ....5000 units
Directions
- Clean the affected area
- Apply small amount of this product(an amount equal to the surface area of the tip of the finger on the area 1 to 3 times daily
- May be covered with a sterile bandage
Warnings
For External Use Only.
Do not use
- In the eyes
- If you are allergic to any of the ingredients
- Over large areas of the body
- Longer than 1 week unless directed by a doctor
- Ask a doctor before use in case of deep or puncture wounds, animal bites or serious burns
Stop use and ask a doctor if
- The condition persists or gets worse
- A rash or allergic reaction develops
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Centre right away