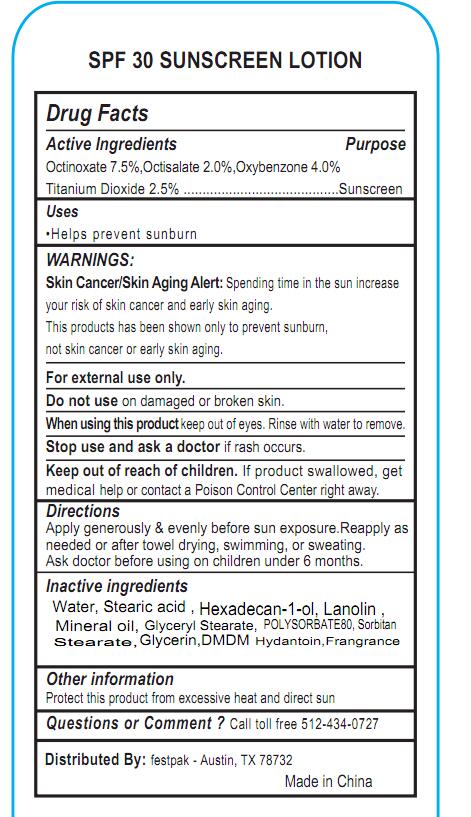
WARNINGS:
Skin Cancer/Skin Aging Alert: Spending time in the sun increase your risk of skin cancer and early skin aging. This products has been shown only to prevent sunburn, not skin cancer or early skin aging. For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs
Keep out of reach of children.
If product swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply generously & evenly before sun exposure.Reapply as needed or after towel drying, swimming, or sweating. Ask doctor before using on children under 6 months.