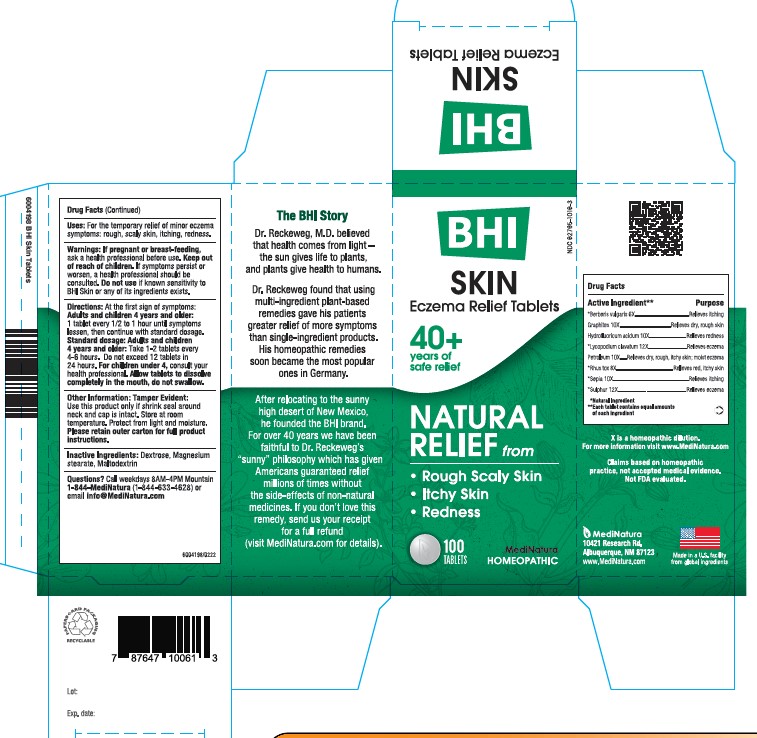
BHI SKIN- berberis vulgaris root bark, graphite, hydrofluoric acid, lycopodium clavatum spore, petrolatum, toxicodendron pubescens leaf, sepia officinalis juice, and sulfur tablet
MediNatura Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS
Each tablet contains: *Berberis vulgaris 6X, Graphites 10X, Hydrofluoricum acidum 10X, *Lycopodium clavatum 12X, *Petroleum 10X, *Rhus toxicodendron 8X, *Sepia 10X, *Sulphur 12X 37.5 mg each.
*Natural Ingredients
INACTIVE INGREDIENTS
Inactive Ingredients: Dextrose, Magnesium Stearate, Maltodextrin
PURPOSE
Eczema Relief Tablets
Relieves:
• Rough Scaly Skin
• Itching Skin
• Redness
USES
For the temporary relief of minor eczema symptoms: rough scaly skin, itching skin, redness
Directions
At first sign of symptoms:Adults and children 4 years and older: 1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 4 years and older: Take 1-2 tablet every 4 to 6 hours. Do not exceed 12 tablets in 24 hours.For children under 4, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.
WARNINGS SECTION
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If symptoms persist or worsen, a health professional should be consulted. Do not use if known sensitivity to BHI Skin or any of its ingredients exists.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
Add image transcription here...
