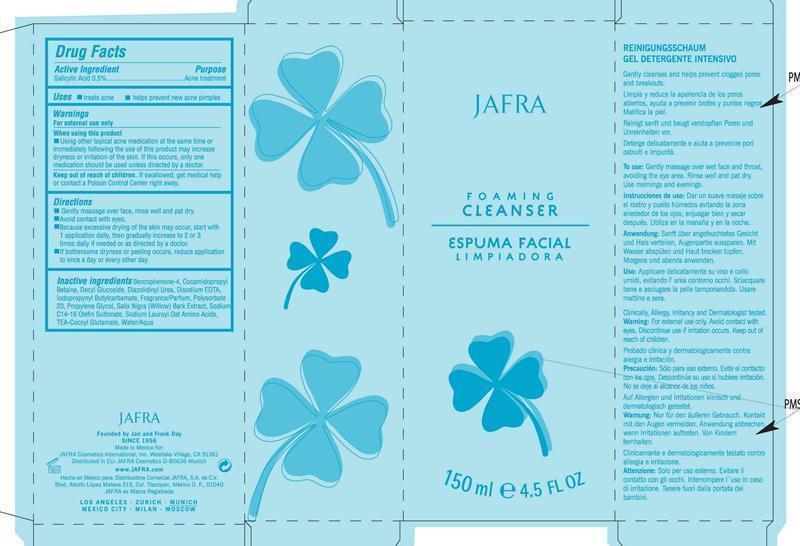
Active Ingredient Purpose
Salicyclic Acid 0.5% Acne Treatment
keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
indications and usage
When using this product
Using other topical acne medication at the same time or immediately following the use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Directions
-Gently massage over face, rinse well and pat dry.
-Avoid contact with eyes.
-Because excessive drying of the skin may occur, start with
1 application daily, then gradually increase to 2 or 3
times daily if needed or as directed by a doctor.
-If bothersome dryness or peeling occurs, reduce application
to once a day or every other day.
Inactive ingredients
Benzophenone-4, cocamidopropyl
Betaine, Decyl Glucoside, Diazolidinyl Urea, Disodium EDTA,
lodopropynyl Butylcarbamate, Fragrance/Parfum, Polysorbate
20, Propylene Glycol, Salix Nigra (Willow) Bark Extract, Sodium
C14-16 Olefin Sulfonate, Sodium Lauroyl Oat Amino Acids,
TEA-Cocoyl Glutamate, Water/Aqua.