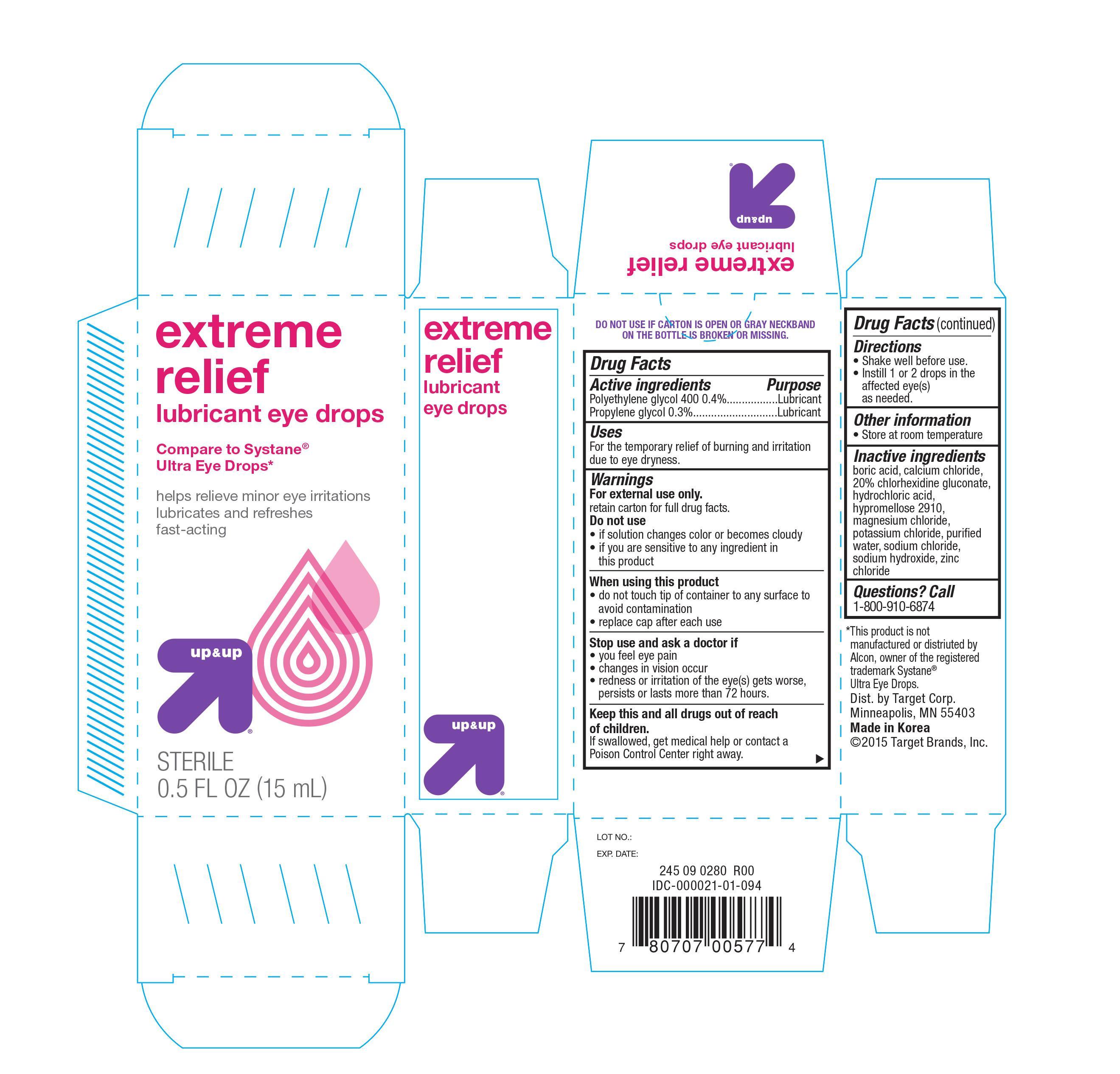
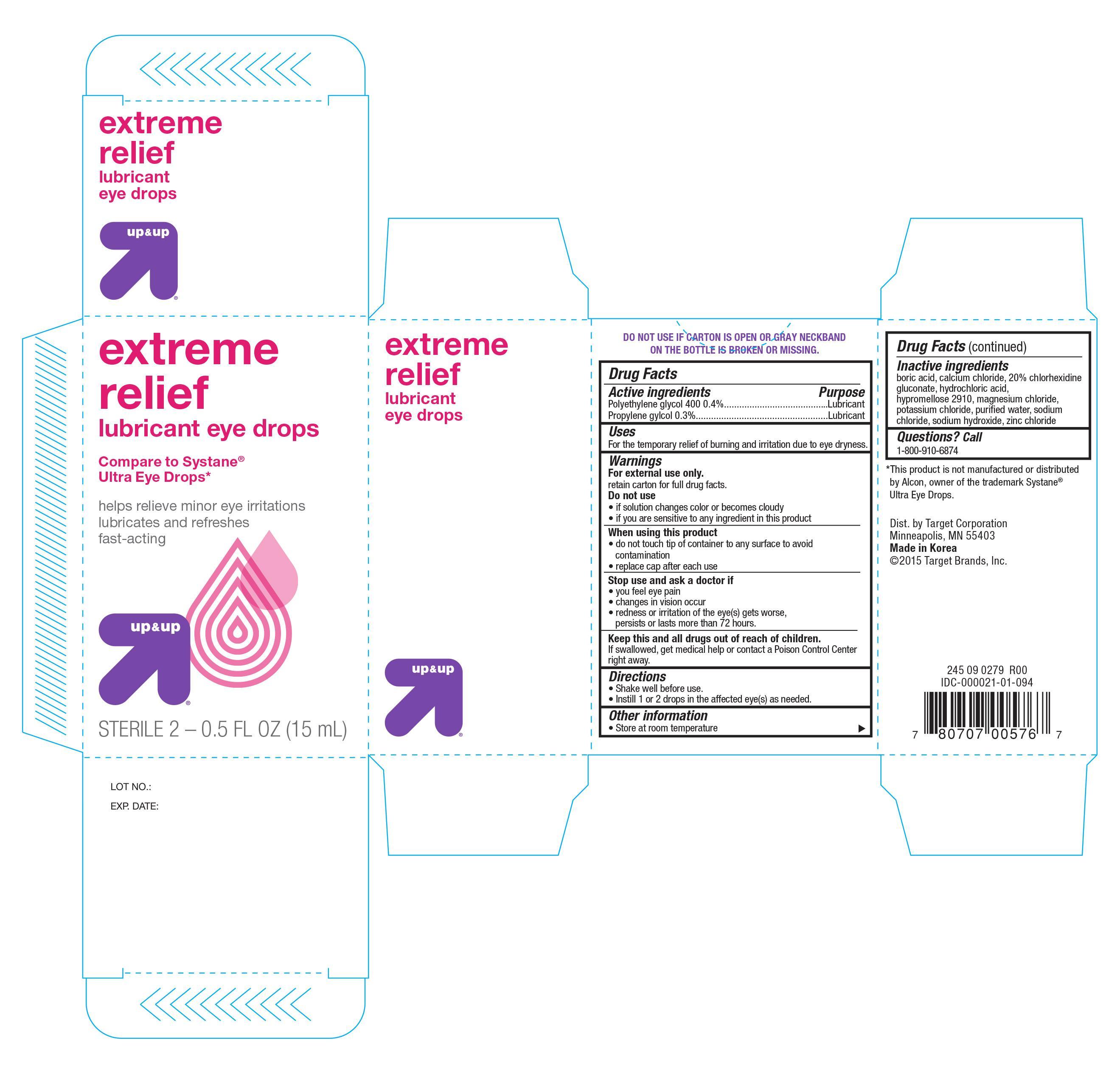
EXTREME RELIEF LUBRICANT- polyethylene glycol 400, and propylene glycol solution/ drops
Target Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients Purpose
Polyethylene glycol 400 0.4%................................... Lubricant
Propylene glycol 0.3%............................................. Lubricant
Uses
For the temporary relief of burning and irritation due to eye dryness
Warnings
For external use only
retain carton for full drug facts
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse, persists or last more than 72 hours
Keep this and all drugs out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
- Shake well before use
- Instill 1 or 2 drops in the affected eye(s) as needed
Other information
- Store at room temperature
Inactive ingredients
boric acid, calcium chloride, 20% chlorhexidine, glyconate, hydrochloric acid, hypromellose 2910, magnesium chloride, potassium chloride, purified water, sodium chloride, sodium hydroxide, zinc chloride
DISTRIBUTED BY:
TARGET CORP.
MINNEAPOLIS, MN 55403
MADE IN KOREA