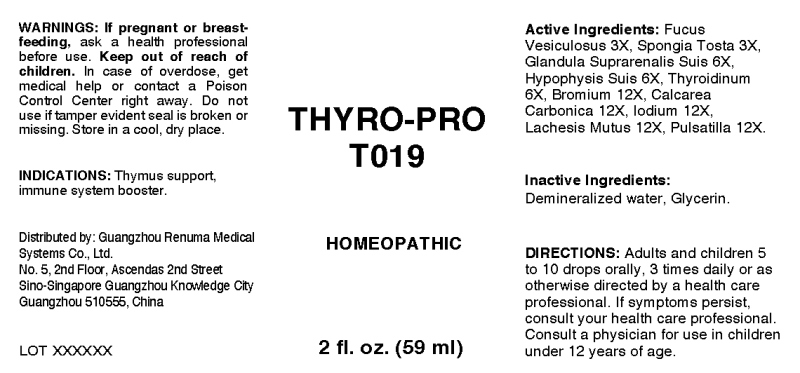
ACTIVE INGREDIENTS:
Fucus Vesiculosus 3X, Spongia Tosta 3X, Glandula Suprarenalis (Suis) 6X, Hypophysis Suis 6X, Bromium 12X, Calcarea Carbonica 12X, Iodium 12X, Lachesis Mutus 12X, Pulsatilla 12X.
WARNINGS:
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.