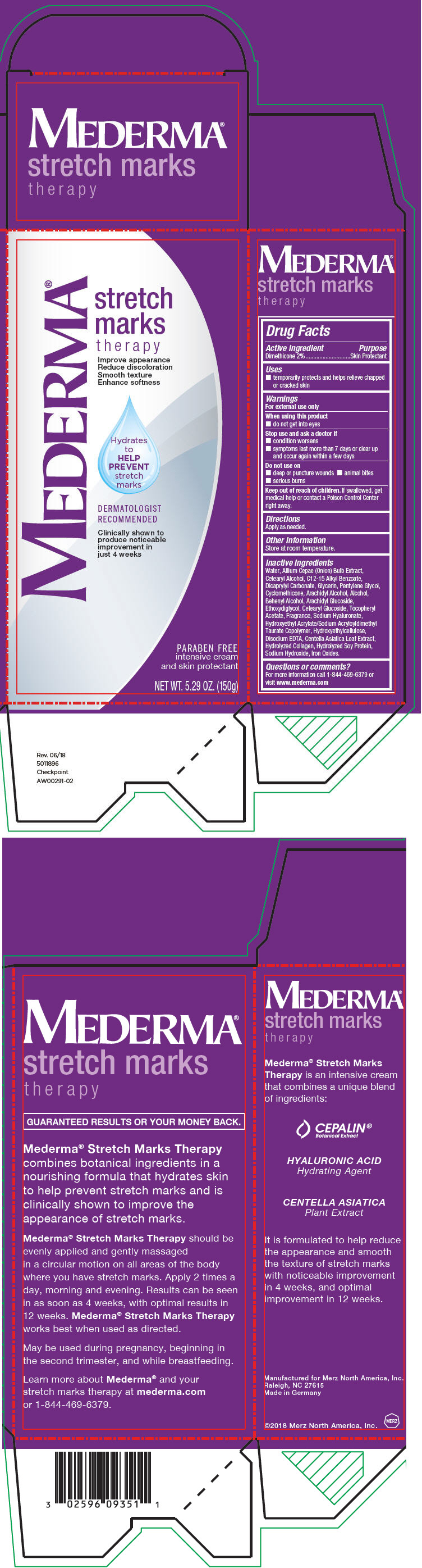
Warnings
For external use only
Inactive Ingredients
Water, Allium Cepae (Onion) Bulb Extract, Cetearyl Alcohol, C12-15 Alkyl Benzoate, Dicaprylyl Carbonate, Glycerin, Pentylene Glycol, Cyclomethicone, Arachidyl Alcohol, Alcohol, Behenyl Alcohol, Arachidyl Glucoside, Ethoxydiglycol, Cetearyl Glucoside, Tocopheryl Acetate, Fragrance, Sodium Hyaluronate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydroxyethylcellulose, Disodium EDTA, Centella Asiatica Leaf Extract, Hydrolyzed Collagen, Hydrolyzed Soy Protein, Sodium Hydroxide, Iron Oxides.
PRINCIPAL DISPLAY PANEL - 150 g Tube Box
MEDERMA®
stretch
marks
therapy
Improve appearance
Reduce discoloration
Smooth texture
Enhance softness
Hydrates
to
HELP
PREVENT
stretch
marks
DERMATOLOGIST
RECOMMENDED
Clinically shown to
produce noticeable
improvement in
just 4 weeks
PARABEN FREE
intensive cream
and skin protectant
NET WT. 5.29 OZ. (150g)