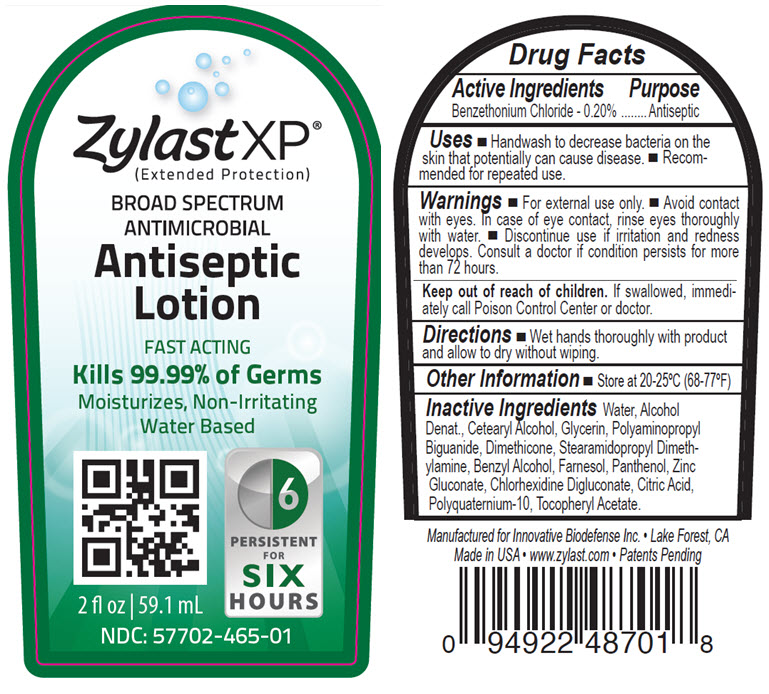
Uses
- Handwash to decrease bacteria on the skin that potentially can cause disease.
- Recommended for repeated use.
Warnings
- For external use only.
- Avoid contact with eyes. In case of eye contact, rinse thoroughly with water.
- Discontinue use if irritation and redness develops. Consult a doctor if condition persists for more than 72 hours.
- If swallowed, immediately call Poison Control Center or doctor.
Inactive ingredients
Water, Alcohol Denat., Cetearyl Alcohol, Glycerin, Polyaminopropyl Biguanide, Dimethicone, Stearamidopropyl Dimethylamine, Benzyl Alcohol, Farnesol, Panthenol, Zinc Gluconate, Chlorhexidine Digluconate, Citric Acid, Polyquaternium-10, Tocopheryl Acetate.
Uses
- Handwash to decrease bacteria on the skin that potentially can cause disease.
- Recommended for repeated use.
Warnings
- For external use only.
- Avoid contact with eyes. In case of eye contact, rinse thoroughly with water.
- Discontinue use if irritation and redness develops. Consult a doctor if condition persists for more than 72 hours.
- If swallowed, immediately call Poison Control Center or doctor.
Inactive Ingredients
Water, Alcohol Denat., Cetearyl Alcohol, Glycerin, Polyaminopropyl Biguanide, Dimethicone, Stearamidopropyl Dimethylamine, Benzyl Alcohol, Farnesol, Panthenol, Zinc Gluconate, Chlorhexidine Digluconate, Citric Acid, Polyquaternium-10, Tocopheryl Acetate.
Package/Label Principal Display Panel
NDC 57702-465-14
Zylast XP
Extended Protection
Broad Spectrum
Antimicrobial
Antiseptic
1000mL (33.8oz)