WARNINGS
For external use only.
Stop use and consult a doctor if the condition persists or gets worse.
Do not use longer than 1 week unless directed by a physician. In case of deep or puncture wounds, animal bites, or serious burn, consult a physician.
Keep out of reach of children. if swallowed get medical help or contact Poison control Center right away.
Keep out of reach of children
Keep out of reach of children. if swallowed get medical help or contact Poison control Center right away.
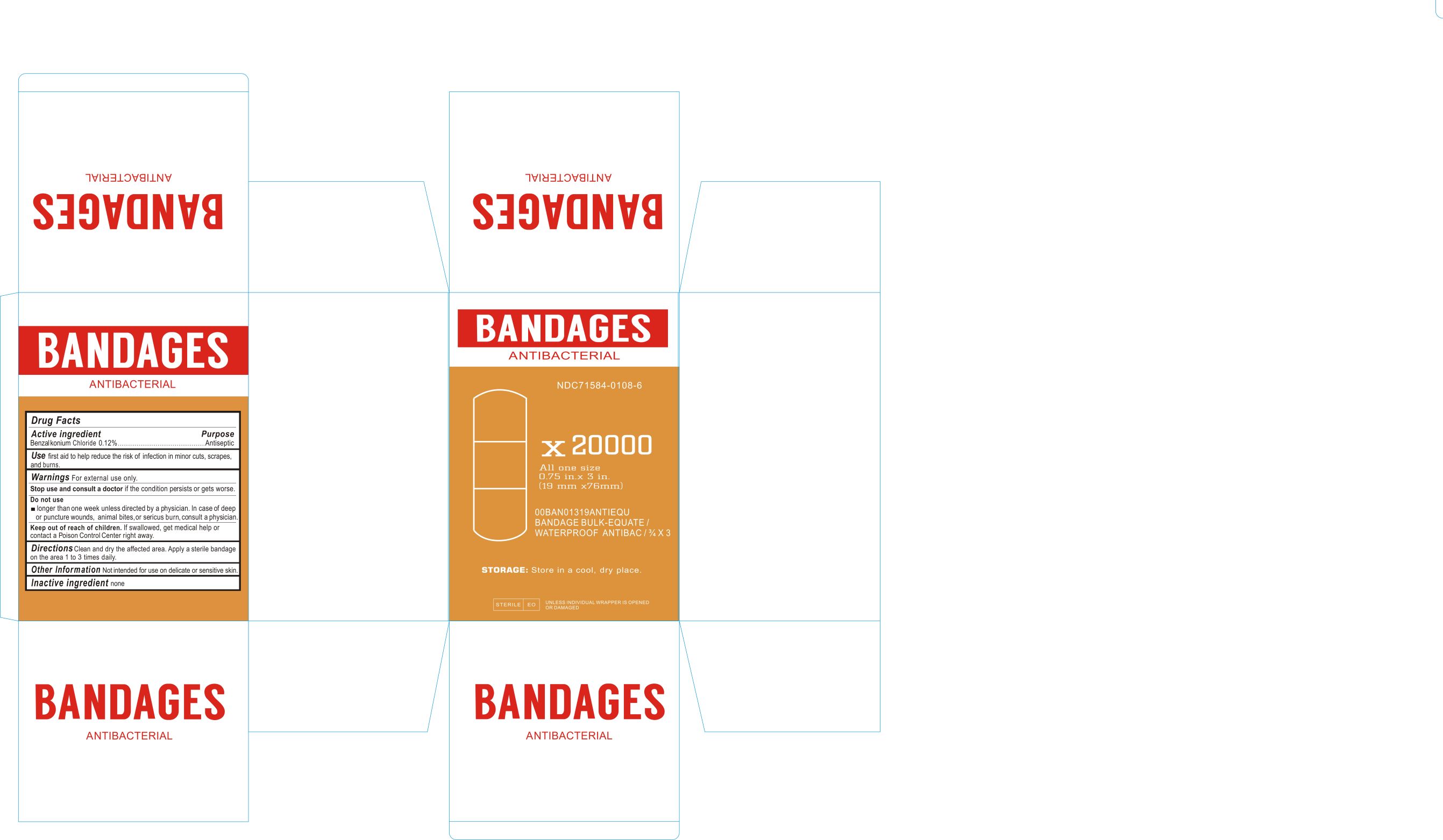
Label principal display panel
NDC 71584-0108-1
ANTIBACTERIAL BANDAGES
Drug Facts
ACTIVE INGREDIENTS
Benzalkonium Chloride 0.12%
Purpose
Antiseptic
USE
First aid to help reduce the risk of infection in minor cuts, scrapes, and burns.
WARNINGS For external use only.
Stop use and consult a doctor if the condition persists or gets worse.
Do not use
longer than one week unless directed by a physician. In case of deep or puncture wounds, animal bites, or serious burn, consult a physician.
Keep out of reach of children. if swallowed get medical help or contact Poison control Center right away.
DIRECTIONS
Clean and dry the affected area. Apply a sterile bandage on the area 1 to 3 times daily.
Other Information: not intended for use on delicate or sensitive skin.
INACTIVE INGREDIENT
None