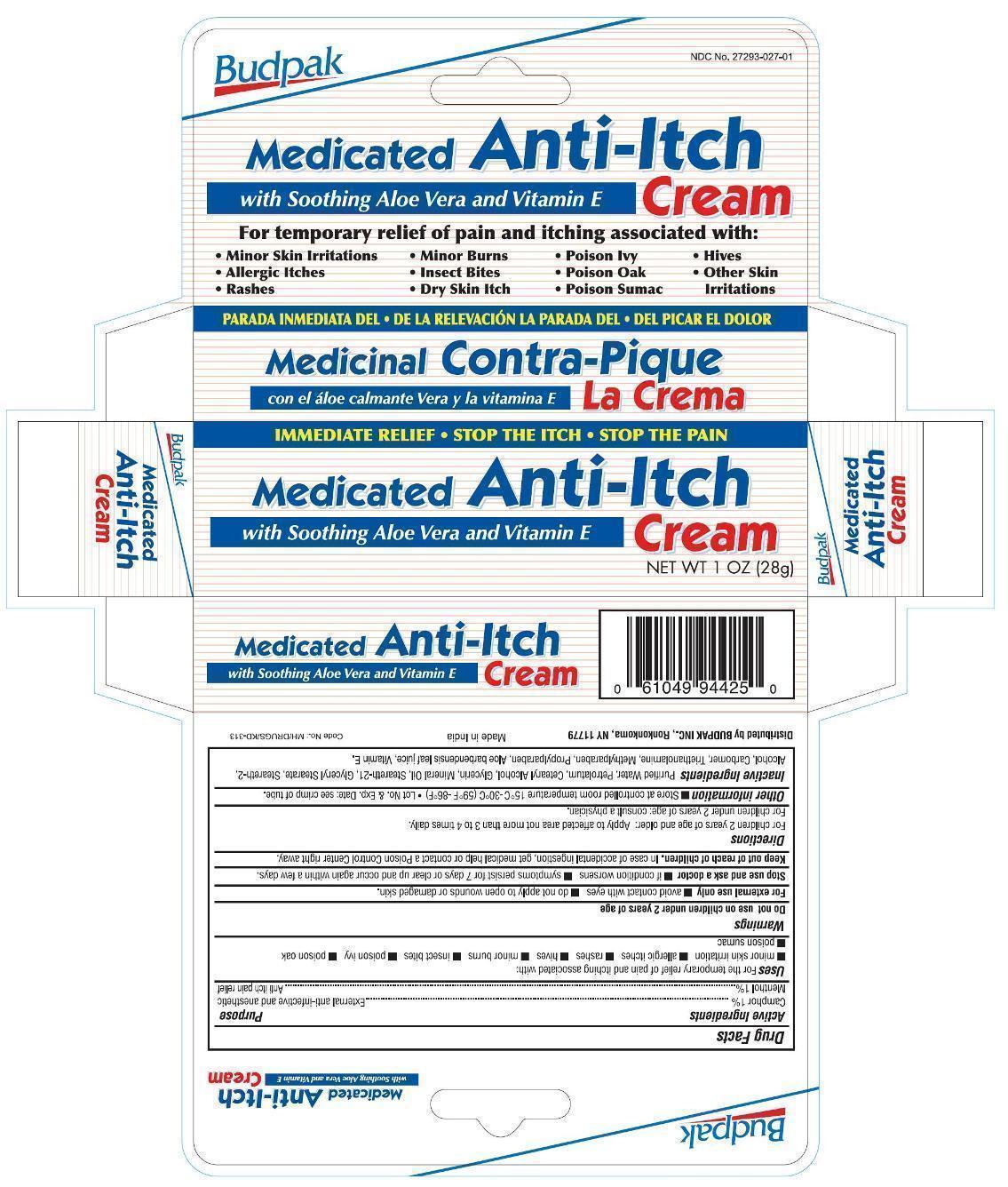
Uses
For the temporary relief of pain and itching associated with:
- minor skin irritation
- allergic itches
- rashes
- hives
- minor burns
- insect bites
- poison ivy
- poison oak
- poison sumac
Do not use on children under 2 years of age.
- avoid contact with eyes
- do not apply to open wound or damaged skin.
Stop use and ask a doctor
- if condition worsens
- symptoms persist for 7 days or clear up and occur again within a few days.
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Directions
-
For children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
-
For children under 2 years of age: consult a physician.
Other information
- Store at controlled room temperature 15ºC to 30ºC (59ºF to 86ºF)
- Lot No. & Exp. Date: see crimp of tube.