HAND SANITIZER- hand sanitizer gel
Tropical Enterprises International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
Ethyl Alcohol 70% v/v
DOSAGE AND ADMINISTRATION
Directions: Wet hands thoroughly with product and allow to dry without wiping.
INACTIVE INGREDIENT
InActive Ingredients: Water, Carbomer, Triethanolamine
INDICATIONS AND USAGE
Use Hand Sanitizer to help reduce bacteria that potentially can cause disease.
For use when soap and water are not available.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. If swallowed, seek prompt medical attention.
OTC PURPOSE
Purpose... Antiseptic
WARNING
WARNINGS: For external use only
When using this product avoid contact with eyes. If contact with eyes occurs, flush with water.
Stop use and ask a doctor if irritation or redness develops and persists. If swallowed, seek prompt medical attention.
Other information: Non-staining, may discolor certain fabrics.
Flammable: Keep away from open flame.
Avoid freezing and excessive heat above 40C
(104F).
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
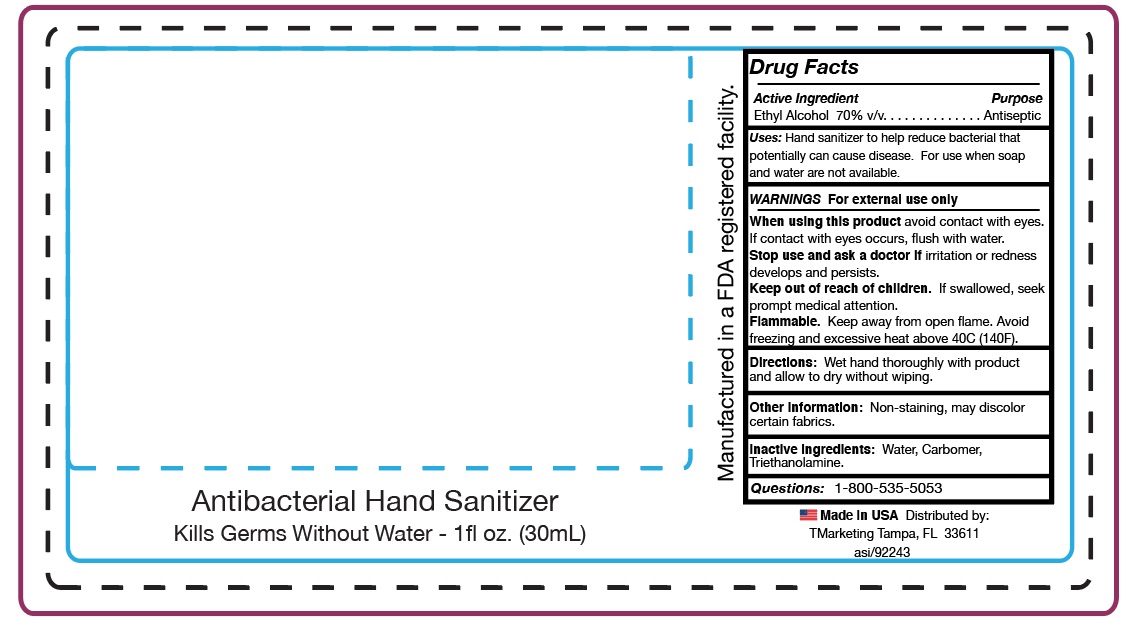
Tropical Enterprises International, Inc.
