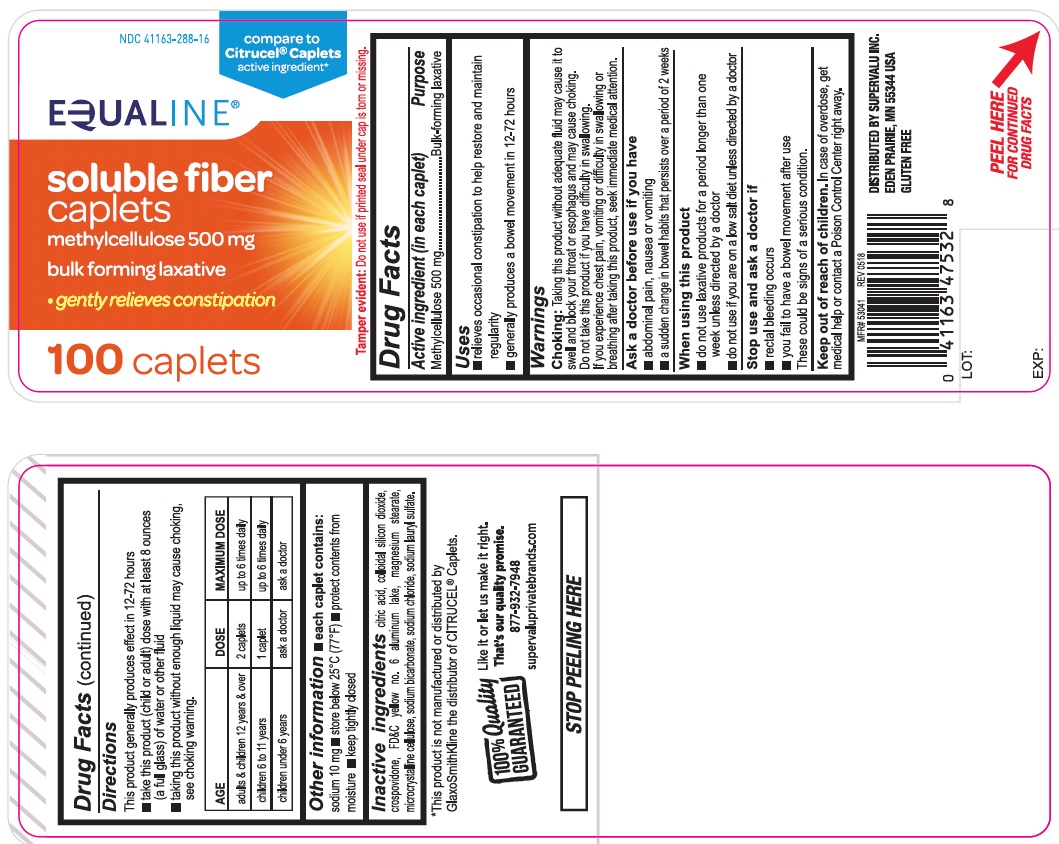
USE(S)
- relieves occasional constipation to help restore and maintain regularity
- generally produces a bowel movement in 12-72 hours
WARNINGS
Choking: Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
ASK A DOCTOR BEFORE USE IF YOU HAVE
- abdominal pain, nausea or vomiting
- a sudden change in bowel habits that persists over a period of 2 weeks
WHEN USING THIS PRODUCT
- do not use laxative products for a period longer than one week unless directed by a doctor.
- do not use if you are on a low salt diet unless directed by a doctor.
STOP USE AND ASK DOCTOR IF
- rectal bleeding occurs
- you fail to have a bowel movement after use
These could be signs of a serious condition
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away
DIRECTIONS
This product generally produces effect in 12-72 hours
- take this product (child or adult) dose with atleast 8 ounces (a full glass) of water or other fluid
- taking this product without enough liquid may cause choking, see choking warning.
| AGE | DOSE | MAXIMUM DOSE |
| adults & children 12 years & over | 2 caplets | up to 6 times daily |
| children 6 to 11 years | 1 caplet | up to 6 times daily |
| children under 6 years | ask a doctor | ask a doctor |
OTHER INFORMATION
- each caplet contains: sodium 10 mg
- store below 25oC (77oF)
- protect contents from moisture
- keep tightly closed