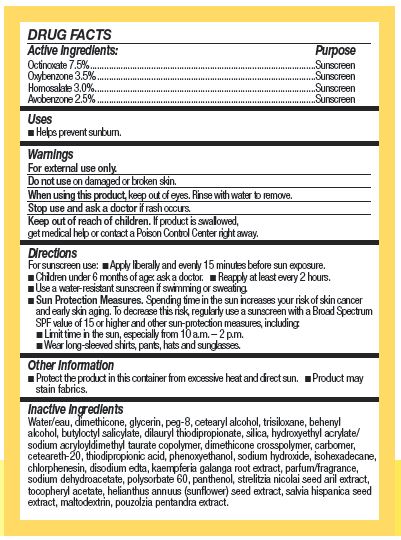
Active Ingredients:
OCTINOXATE 7.5 % ……………………………
OXYBENZONE 3.5 % ……………………………
HOMOSALATE 3.0 % ………………………………
AVOBENZONE 2.5 % ……………………………
Keep our of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
For suncreen use:
Apply liberally and evenly 15 minutes before sun exposure.
Children under 6 months of age: ask a doctor.
Reapply at least every 2 hours.
Use a water-resistant sunscreen if swimming or sweating.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun-procteion measures, including:
Limite time in the sun, expecially from 10 a.m.-2 p.m.
Wear long-sleeved shirts, pants, hats and sunglasses.
Other Information
- Protect the product in this container from excessive heat and direct sun,
- Product may stain fabrics.
Inactive ingredients
WATER/EAU, DIMETHICONE, GLYCERIN, PEG-8, CETEARYL ALCOHOL, TRISILOXANE, BEHENYL ALCOHOL, BUTYLOCTYL SALICYLATE, DILAURYL THIODIPROPIONATE, SILICA, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, DIMETHICONE CROSSPOLYMER, CARBOMER, CETEARETH-20, THIODIPROPIONIC ACID, PHENOXYETHANOL, SODIUM HYDROXIDE, ISOHEXADECANE, CHLORPHENESIN, DISODIUM EDTA, KAEMPFERIA GALANGA ROOT EXTRACT, PARFUM/FRAGRANCE, SODIUM DEHYDROACETATE, POLYSORBATE 60, PANTHENOL, STRELITZIA NICOLAI SEED ARIL EXTRACT, TOCOPHERYL ACETATE, HELIANTHUS ANNUUS (SUNFLOWER) SEED EXTRACT, SALVIA HISPANICA SEED EXTRACT, MALTODEXTRIN, POUZOLZIA PENTANDRA EXTRACT.