Warnings
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
Directions
- dosage should be taken one hour before travel starts
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- adults and children 12 years and over: take 1 to 2 chewable tablets once daily or as directed by a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from heat and humidity
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, FD&C red #40 aluminum lake, flavor, lactose anhydrous, magnesium stearate, saccharin sodium, silicon dioxide
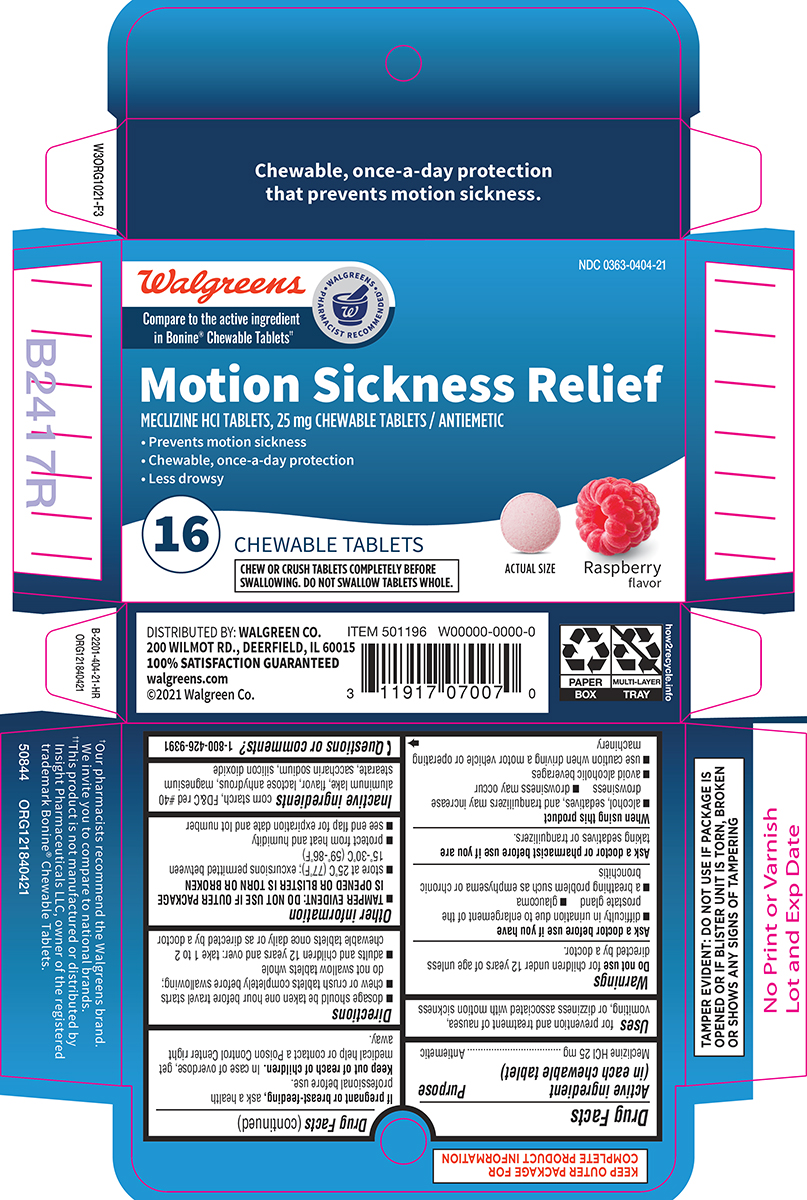
Principal Display Panel
Walgreens
Compare to the active ingredient
in Bonine® Chewable Tablets††
WALGREENS PHARMACIST RECOMMENDED†
NDC 0363-0404-21
Motion Sickness Relief
MECLIZINE HCl TABLETS, 25 mg CHEWABLE TABLETS / ANTIEMETIC
• Prevents motion sickness
• Chewable, once-a-day protection
• Less drowsy
16 CHEWABLE TABLETS
CHEW OR CRUSH TABLETS COMPLETELY BEFORE
SWALLOWING. DO NOT SWALLOW TABLETS WHOLE.
ACTUAL SIZE
Raspberry
flavor
Chewable, once-a-day protection
that prevents motion sickness.
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING
†Our pharmacists recommend the Walgreens brand.
We invite you to compare to national brands.
††This product is not manufactured or distributed by
Insight Pharmaceuticals LLC, owner of the registered
trademark Bonine® Chewable Tablets.
50844 ORG121840421
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com
©2021 Walgreen Co.
Walgreens 44-404