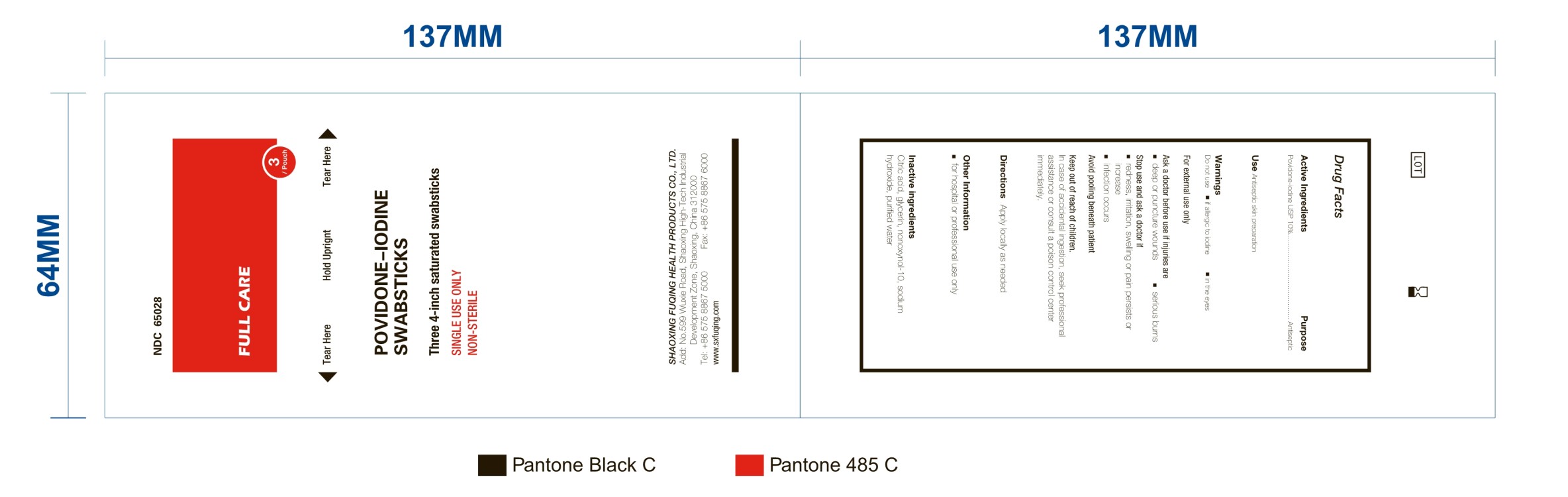
POVIDONE IODINE SWABSTICKS- povidone-iodine swab
Shaoxing Fuqing Health Products Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient: Povidone-Iodine USP (10%)
Use: Antiseptic skin preparation
Warnings:
Do not use
- if allergic to iodine
- in the eyes
For external use only
Avoid "pooling" beneath patients.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or consult a poison control center immediately
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling, or pain persists or increases
- infection occurs
Other information
- For hospital or professional use only
Inactive Ingredient
Citric Acid, Glycerin, Nonoxyol-10, Sodium Hydroxide, Water
Shaoxing Fuqing Health Products Co., Ltd.