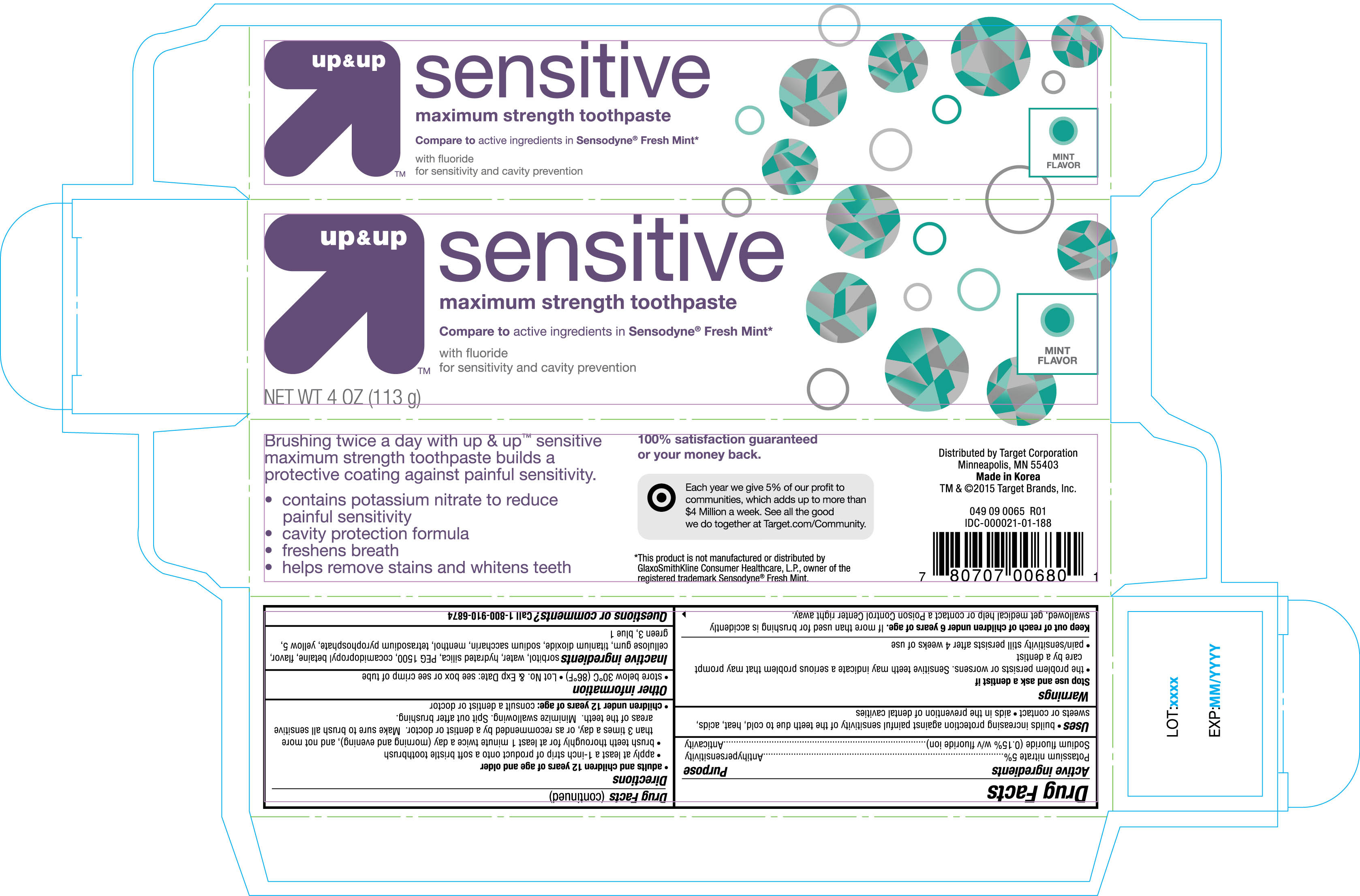
Active ingredients Purpose
Potassium nitrate 5%.......................................... Antihypersensitivity
Sodium fluoride (0.15% w/v fluoride ion)............................ Anticavity
Uses
- builds increasing protection against painful sensitivty of the teeth due to cold, heat, acids, sweets or contact
- aids in the prevention of dental cavities
Warnings
Stop use and ask a dentist if
- the problem persists or worsens. Sensitive teeth may indicate a serious problem that may prompt care by a dentist
- pain/sensitivity still persists after 4 weeks of use
Keep out of reach of children under 6 years of age. If more than used for brushing is accidently swallowed, get medical help or contact a Posion Control Center right away.
Directions
- adults and children 12 years of age and older
- apply at least 1-inch strip of product onto a soft bristle toothbrush
- brush teeth thoroughly for at least 1 minute twice a day (morning and evening), and not more than 3 times a day, or as recommended by a dentist or doctor. Make sure to brush all sensitive areas of the teeth. Minimize swallowing. Spit out after brushing.
- children under 12 years of age: consult a dentist or doctor