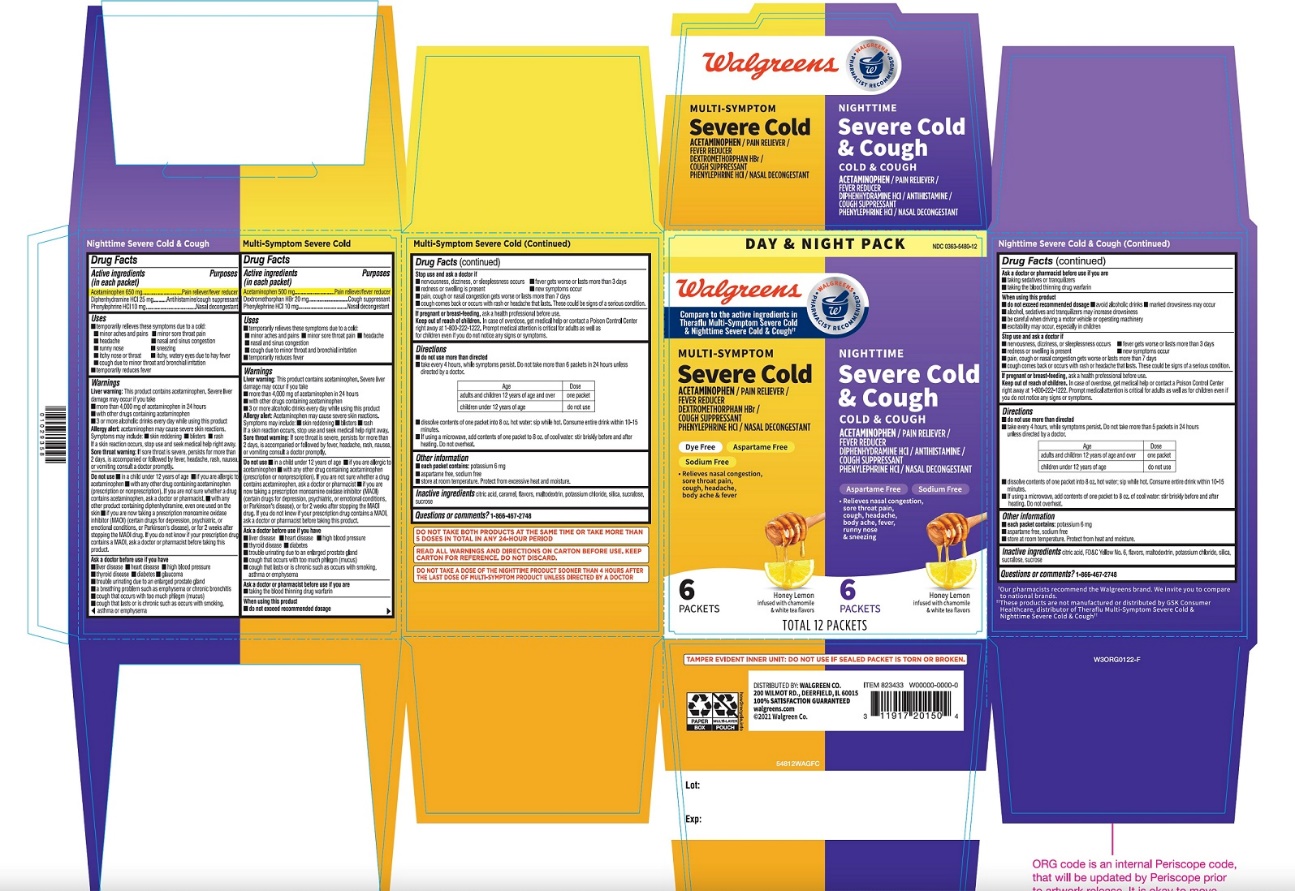
Active ingredients (in each packet) – Multi Symptom Severe Cold
Acetaminophen 500 mg
Dextromethorphan hydrobromide 20 mg
Phenylephrine hydrochloride 10 mg
Active ingredients (in each packet) - Nighttime Severe Cold and Cough
Acetaminophen 650 mg
Diphenhydramine hydrochloride 25 mg
Phenylephrine hydrochloride 10 mg
Purpose - Multi Symptom Severe Cold
Pain reliever/fever reducer
Cough Suppressant
Nasal Decongestant
Purpose - Nighttime Severe Cold and Cough
Pain reliever/fever reducer
Antihistamine/Cough suppressant
Nasal decongestant
Uses - Multi Symptom Severe Cold
- •
- temporarily relieves these symptoms due to a cold:
- •
- minor aches and pains
- •
- minor sore throat pain
- •
- headache
- •
- nasal and sinus congestion
- •
- cough due to minor throat and bronchial irritation
- •
- temporarily reduces fever
Uses - Nighttime Severe Cold and Cough
- •
- temporarily relieves these symptoms due to a cold:
- •
- minor aches and pains
- •
- minor sore throat pain
- •
- headache
- •
- nasal and sinus congestion
- •
- runny nose
- •
- sneezing
- •
- itchy nose or throat
- •
- itchy, watery eyes due to hay fever
- •
- cough due to minor throat and bronchial irritation
- •
- temporarily reduces fever
Warnings – Multi Symptom Severe Cold /Nighttime Severe Cold and Cough
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 4,000 mg of acetaminophen in 24 hours
- •
- with other drugs containing acetaminophen
3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use - Multi Symptom Severe Cold
- •
- in a child under 12 years of age
- •
- if you are allergic to acetaminophen
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Do not use - Nighttime Severe Cold and Cough
- •
- in a child under 12 years of age
- •
- if you are allergic to acetaminophen
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
- •
- with any other product containing diphenhydramine, even one used on skin
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have - Multi Symptom Severe Cold
- •
- liver disease
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
Ask a doctor before use if you have - Nighttime Severe Cold and Cough
- •
- liver disease
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- glaucoma
- •
- trouble urinating due to an enlarged prostate gland
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
Ask a doctor or pharmacist before use if you are - Multi Symptom Severe Cold
taking the blood thinning drug warfarin
Ask a doctor or pharmacist before use if you are - Nighttime Severe Cold and Cough
- •
- taking sedatives or tranquilizers
- •
- taking the blood thinning drug warfarin
When using this product - Nighttime Severe Cold and Cough
- •
- do not exceed recommended dosage
- •
- avoid alcoholic drinks
- •
- marked drowsiness may occur
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
- •
- excitability may occur, especially in children
Stop use and ask a doctor if – Multi Symptom Severe Cold /Nighttime Severe Cold and Cough
- •
- nervousness, dizziness, or sleeplessness occurs
- •
- fever gets worse or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- pain, cough or nasal congestion gets worse or lasts more than 7 days
- •
- cough comes back or occurs with fever, rash or headache that lasts. These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions – Multi Symptom Severe Cold
- •
- do not use more than directed
- •
- take every 4 hours, while symptoms persist .do not take more than 6 packets in 24 hours unless directed by a doctor
|
Age |
Dose |
|
Adults and children 12 years of age |
One packet |
|
Children under 12 years of age |
Do not use |
- •
- dissolve contents of one packet into 8 oz. hot water: sip while hot. Consume entire drink within 10 - 15 minutes.
- •
- if using a microwave, add contents of one packet to 8 oz. of cool water: stir briskly before and after heating, Do not overheat
Directions Nighttime Severe Cold and Cough
- •
- do not use more than directed
- •
- take every 4 hours while symptoms persist, not to exceed 5 packets in 24 hours unless directed by a doctor
|
Age |
Dose |
|
Adults and Children 12 years of age and over |
One packet |
|
Children under 12 years of age |
do not use |
- •
- dissolve contents of one packet into 8 oz. hot water: sip while hot. Consume entire drink within 10 - 15 minutes.
- •
- if using a microwave, add contents of one packet to 8 oz. of cool water: stir briskly before and after heating, Do not overheat.
Other information - Multi Symptom Severe Cold
- •
- each packet contains: potassium 6 mg
- •
- store at room temperature. Protect from excessive heat and moisture.
Other information - Nighttime Severe Cold and Cough
- •
- each packet contains: potassium 6 mg
- •
- store at room temperature. Protect from excessive heat and moisture
Inactive ingredients - Multi Symptom Severe Cold
citric acid, caramel, flavors, maltodextrin, potassium chloride, silica ,sucralose, sucrose
Inactive ingredients - Nighttime Severe Cold and Cough
citric acid, F D & C yellow #6 , flavors, maltodextrin, potassium chloride, silica ,sucralose, sucrose,
Package/Label Principal Display Panel
Walgreens
DAY & NIGHT PACK
Compare to Theraflu® Multi Symptom Severe Cold and Nighttime Severe Cold & Cough active ingredients††
MULTI- SYMPTOM
Wal-Flu®
Severe
COLD
ACETAMINOPHEN / PAIN RELIEVER/ FEVER REDUCER
DEXTROMETHORPHAN HBr / COUGH SUPPRESSANT
PHENYLEPHRINE HCl / NASAL DECONGESTANT
ASPARTAME FREE
SODIUM FREE
- •
- Relieves nasal congestion, sore throat pain, cough, headache, body ache & fever
GREEN TEA & HONEY LEMON FLAVORS
INFUSED WITH MENTHOL AND GREEN TEA FLAVOR
- 6 PACKETS
NIGHTTIME
Wal-Flu®
Severe
COLD & COUGH
ACETAMINOPHEN/ PAIN RELIEVER/ FEVER REDUCER
DIPHENHYDRAMINE HCl / ANTIHISTAMINE/ COUGH SUPPRESSANT
PHENYLEPHRINE HCl / NASAL DECONGESTANT
ASPARTAME FREE
SODIUM FREE
Relieves nasal congestion, sore throat pain, cough, headache, body ache & fever, runny nose
& sneezing
HONEY LEMON INFUSED WITH CHAMOMILE &WHITE TEA FLAVORS
6 PACKETS
TOTAL 12 PACKETS
|
READ ALL WARNINGS AND DIRECTIONS ON CARTON BEFORE USE .KEEP CARTON FOR REFERENCE, DO NOT DISCARD. |
|
DO NOT TAKE BOTH PRODUCTS AT THE SAME TIME OR TAKE MORE THAN 5 DOSES IN TOTAL IN ANY 24 –HOUR PERIOD. |
|
DO NOT TAKE A DOSE OF THE NIGHTTIME PRODUCT SOONER THAN 4 HOURS AFTER THE LAST DOSE OF DAYTIME PRODUCT UNLESS DIRECTED BY DOCTOR. |
††These Products are not manufactured or distributed by GSK Consumer Healthcare, distributor of Theraflu® Daytime Severe Cold & Cough and Theraflu ®Nighttime Severe Cold & Cough
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
Walgreens 100% SATISFACTION GUARANTEED
Walgreens.com ©2018 Walgreen Co,
WALGREENS PHARMACIST RECOMMENDED‡
Walgreens Pharmacist Survey
|
TAMPER EVIDENT INNER UNIT: DO NOT USE IF SEALED PACKET IS TORN OR BROKEN |