DEVICE DESCRIPTION
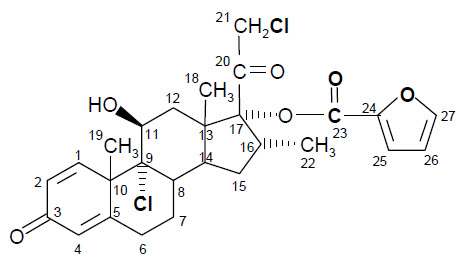
The Propel/Propel mini/Propel Contour sinus implant is a bioabsorbable implant designed to maintain patency of the sinus cavity (Propel/Propel mini) or sinus ostium (Propel Contour). The Propel/Propel mini/Propel Contour implant is manufactured from a synthetic bioabsorbable copolymer, poly (L-lactide-co-glycolide) (PLG). The implant contains mometasone furoate (active ingredient), a synthetic corticosteroid with anti-inflammatory activity. Mometasone furoate is a white to off-white powder. The chemical name is 9α,21-dichloro-11β,17α-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), with the empirical formula C27H30Cl2O6, and a molecular weight of 521.43 g/mol. Mometasone furoate is a hydrophobic drug that is practically insoluble in water. Mometasone furoate is stable under aqueous, acidic and oxidative conditions. Mometasone furoate can degrade under extreme basic, thermal and photolytic conditions. The chemical structure is shown. The drug is embedded in a bioabsorbable polymer matrix containing poly-(DL-lactide-co-glycolide) and polyethylene glycol (inactive ingredients) which provides for gradual release of the drug.
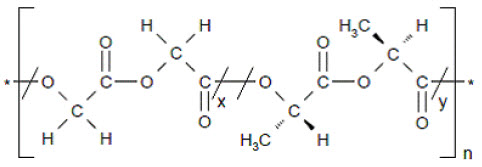
The inactive ingredients on the sinus implant are poly-(DL-lactide-co-glycolide) and polyethylene glycol. Poly-(DL-lactide-co-glycolide) is an amorphous biodegradable polymer. The chemical structure is shown below.
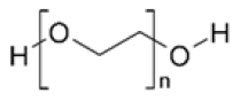
Polyethylene glycol is a hydrophilic polyether compound that is highly flexible. It is non-toxic and non-immunogenic. The chemical structure is shown below.
The implant is designed to accommodate the size and variability of the post-surgical ethmoid sinus anatomy (for Propel), ethmoid or frontal sinus anatomy (for Propel mini), and frontal or maxillary sinus ostium anatomy for Propel Contour. The Propel/Propel mini/Propel Contour implant is designed to be inserted by a physician under endoscopic visualization and once inserted, the implant is designed to be self-retaining against the mucosa of the surgically enlarged sinus (Propel/Propel mini) or sinus ostium (Propel Contour). A delivery system is provided to access the ethmoid sinus (for Propel), ethmoid or frontal sinus (for Propel mini), and frontal or maxillary sinus ostium (for the Propel Contour) and insert the implant.
INDICATIONS FOR USE
The Propel sinus implant is intended for use in patients ≥ 18 years of age following ethmoid sinus surgery to maintain patency, thereby reducing the need for post-operative intervention such as surgical adhesion lysis and/or use of oral steroids. The Propel implant separates mucosal tissues, provides stabilization of the middle turbinate, prevents obstruction by adhesions, and reduces edema.
The Propel mini sinus implant is intended for use in patients ≥ 18 years of age following ethmoid/frontal sinus surgery to maintain patency of the ethmoid sinus or frontal sinus opening. The Propel mini sinus implant separates/dilates surrounding mucosal tissues, provides stabilization of the middle turbinate, prevents obstruction by adhesions, and reduces inflammation. The implant reduces the need for post-operative intervention such as surgical adhesion lysis and/or use of oral steroids.
The Propel Contour sinus implant is intended for use in patients ≥ 18 years of age to maintain patency of the frontal and maxillary sinus ostia following sinus surgery and locally deliver steroids to the sinus mucosa. The PROPEL Contour sinus implant separates/dilates mucosal tissues, prevents obstruction by adhesions/scarring, and reduces edema. The implant reduces the need for post-operative intervention such as surgical adhesion lysis and/or use of oral steroids.
CONTRAINDICATIONS
The use of the Propel/Propel mini/Propel Contour sinus implant is contraindicated in the following patients:
•Patients with suspected or confirmed hypersensitivity and / or intolerance to mometasone furoate.
•Patients with a known hypersensitivity to lactide, glycolide or caprolactone copolymers.
WARNINGS AND PRECAUTIONS
WARNINGS
- •
- The Propel/Propel mini/Propel Contour sinus implant is designed for single patient use only. Do not reprocess or reuse.
- •
- Do not use if the package is open or damaged.
PRECAUTIONS
- •
- Special care should be taken to avoid bending, twisting or damaging the implant.
- •
- The implant is not designed to be modified by the physician.
- •
- The implant is not intended to be compressed and loaded into the delivery system more than two times.
- •
- The implant must be placed under endoscopic visualization.
- •
- The implant exhibits no antimicrobial properties.
- •
- Foreign body reaction may occur as is possible with most surgical adjuncts.
- •
- In rare instances, the physiochemical condition associated with sinus surgery, both with and without sinus implants or packing, may present a risk of toxic shock syndrome (TSS).
- •
- Pediatric Use: The safety and effectiveness of the implant in pediatric patients have not been established.
- •
- Pregnancy and Nursing Females: The safety and effectiveness of the implant in pregnant or nursing females have not been established.
DRUG INFORMATION
MECHANISM OF ACTION: Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. The precise mechanism behind the anti-inflammatory properties of the eluted mometasone furoate is not known.
PHARMACOKINETICS: The Propel sinus implant underwent pharmacokinetics testing. Following bilateral drug-eluting Propel implant placement after sinus surgery for chronic sinusitis and subsequent weekly morning blood sampling for 4 weeks in 5 adult patients, plasma mometasone furoate concentrations were not quantifiable at any time point. Mean cortisol concentrations were within normal limits.
DRUG INTERACTIONS
No drug-drug interaction studies have been conducted with the Propel/Propel mini/Propel Contour implant.
CARCINOGENICITY, GENOTOXICITY AND REPRODUCTIVE TOXICITY
No long term studies in animals have been performed to evaluate the carcinogenic potential of the Propel/Propel mini/Propel Contour implant.
DOSAGE AND ADMINISTRATION
Each Propel/Propel mini/Propel Contour sinus implant contains 370µg of mometasone furoate which is gradually released over time.
I. POTENTIAL ADVERSE EFFECTS OF THE DEVICE ON HEALTH
Potential Adverse Effects: Potential adverse effects associated with the Propel/Propel mini/Propel Contour sinus implant are anticipated to be similar to those associated with other sinus stents, gels or packing.
Potential adverse effects associated with the Propel/Propel mini/Propel Contour sinus implant include, but may not be limited to:
- •
- Premature displacement of implant or implant fragments
- •
- Swallowing implant or implant fragments
- •
- Pain/pressure/headache may result from the adherence of crusting to, or presence of the implant
- •
- Aspiration of small implant fragments (not observed in clinical trials)
- •
- Foreign body response, including formation of granulation tissue
Potential risks or side effects associated with intranasal mometasone furoate include:
- •
- nasal irritation
- •
- hypersensitivity reaction
- •
- intranasal bleeding
- •
- localized infection (bacterial, fungal or viral) in the nose or pharynx
- •
- nasal burning
- •
- nasal dryness
- •
- susceptibility to secondary infections due to bacteria, fungi or viruses
- •
- glaucoma/elevation of intraocular pressure
- •
- cataracts/change in lens opacities
- •
- headache
- •
- pharyngitis
Potential risks or general side effects associated with steroids:
- •
- alteration of the HPA axis including growth suppression
- •
- immunosuppression
- •
- hypersensitivity reactions
- •
- headache
- •
- epistaxis
- •
- coughing
- •
- vomiting
- •
- candidiasis
- •
- glaucoma/elevation in intraocular pressure
- •
- cataracts/changes in lens opacities
- •
- arthralgia
- •
- myalgia
Manufactured by:
Intersect ENT Inc.
1555 Adams Drive
Menlo Park, CA 94025
650-641-2100
www.intersectENT.com
Customer Service 1-866-531-6004
CustomerService@intersectENT.com
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – PROPEL

Picture of label is representative of label content
PROPEL
(MOMETASONE FUROATE IMPLANT, 370 µg)
CONTENTS INCLUDE:
One sinus implant (nominal length: 23 mm)
One straight delivery system
Caution: Federal law (US) restricts this product to sale by or on the order of a physician.
|
Reference Number |
Read Instructions Prior to Use |
For Single Use Only |
|
Lot Number |
Sterilized Using Radiation |
Contents are STERILE in unopened and undamaged package |
|
Use By |
Store at Room Temperature (15-30° C) |
intersect® ENT
4282023 10:00:38
00328 Rev. R
1555 Adams Drive, Menlo Park, CA 94025
Customer Service 1-866-531-6004
CustomerService@intersectENT.com
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – PROPEL MINI

PROPEL mini
(MOMETASONE FUROATE IMPLANT, 370 µg)
CONTENTS INCLUDE:
One sinus implant (nominal length: 16 mm)
One delivery system
Caution: Federal law (US) restricts this product to sale by or on the order of a physician.
|
Reference Number |
Read Instructions Prior to Use |
For Single Use Only |
|
Lot Number |
Sterilized Using Radiation |
Contents are STERILE in unopened and undamaged package. |
|
Use By |
Store at Room Temperature (15-30° C) |
intersect® ENT
7312023 13:17:43
00327 Rev. T
1555 Adams Drive, Menlo Park, CA 94025
Customer Service 1-866-531-6004
CustomerService@intersectENT.com
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – PROPEL® CONTOUR

PROPEL® CONTOUR
(MOMETASONE FUROATE IMPLANT, 370 µg)
CONTENTS INCLUDE:
One sinus implant
One delivery system
Caution: Federal law (US) restricts this product to sale by or on the order of a physician.
|
Reference Number |
Read Instructions Prior to Use |
For Single Use Only |
|
Lot Number |
Sterilized Using Radiation |
Contents are STERILE in unopened and undamaged package. |
|
Use By |
Store at Room Temperature (15-30° C) |
intersect® ENT
552023 10:06:31
00385 Rev. M
1555 Adams Drive, Menlo Park, CA 94025
Customer Service 1-866-531-6004
CustomerService@intersectENT.com