POLYETHYLENE GLYCOL 3350- polyethylene glycol 3350 powder, for solution
Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
----------
Drug Facts
Active ingredient (in each dose)
Polyethylene Glycol 3350, 17 g (cap filled to line)
Purpose
Osmotic Laxative
INDICATIONS AND USAGE
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
WARNINGS
Do not use if you are allergic to polyethylene glycol
Do not use if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Ask a doctor of pharmacist before use if you are taking a prescription drug
When using this product you may have loose, watery, more frequent stools
Stop use and ask a doctor if
- you have rectal bleeding or your nausea, bloating or cramping or abdominal pain gets worse. These may be signs of a serious condition.
- you get diarrhea
- you need to use a laxative for longer than 1 week
- side effects occur. You may report side effects to FDA at1-800-FDA-1088
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- fill to top of white section in cap which is marked to indicate the correct dose (17g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use no more than 7 days
- children 16 years of age or under : ask a doctor
- store at 20°-25°C (68°-77°F)
- tamper-evident : do not use if foil seal under cap, printed with "SEALED for YOUR PROTECTION" is missing, open or broken
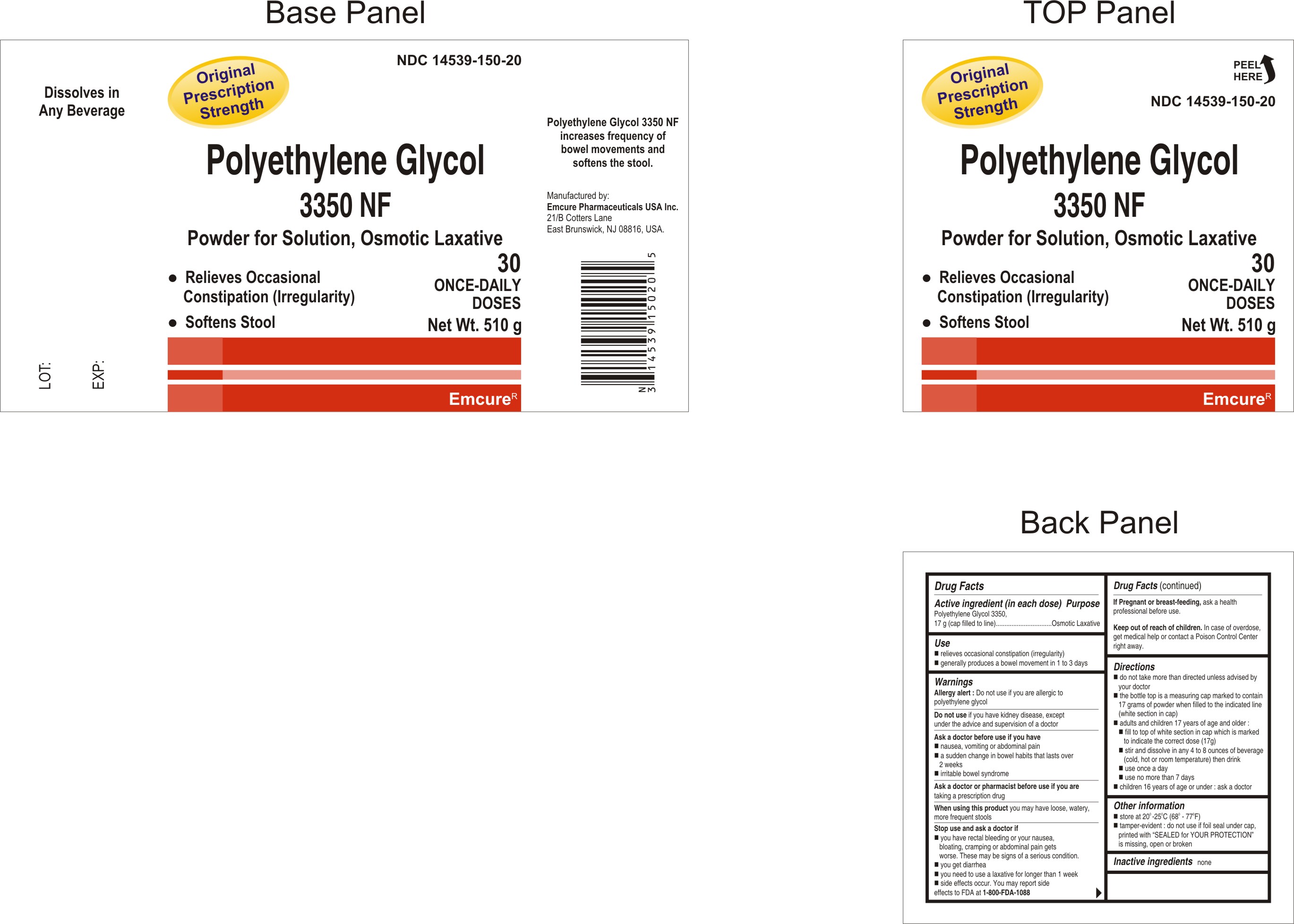
Principal display panel
Polyethylene Glycol 3350 NF
Powder for Solution, Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stools
Original Prescription Strength
Dissolves in Any Beverage
30 ONCE-DAILY DOSES
Net Wt. 510 g
Polyethylene Glycol 3350 NF increases frequency of bowel movements and softens the stools.
Manufactured By:
Emcure Pharmaceuticals USA Inc.
21/B Cotters Lane
East Brunswick, NJ 08816, USA.
Top panel
Polyethylene Glycol 3350 NF
Powder for Solution, Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stools
PEEL HERE
Original Prescription Strength
30 ONCE-DAILY DOSES
Net Wt. 510 g
Drug Facts
Active ingredient (in each dose) Purpose
Polyethylene Glycol 3350,
17 g (cap filled to line).............Osmotic Laxative
Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
Allergy alert: Do not use if you are allergic to polyethylene glycol.
Do not use if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- Nausea, vomiting or abdominal pain
- A sudden change in bowel habits that lasts over 2 weeks
- Irritable bowel syndrome
Ask a doctor or pharmacist before use if you are taking a prescription drug
When using this product you may have loose, watery, more frequent stools
Stop use and ask a doctor if
- you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
- you get diarrhea
- you need to use a laxative for longer than 1 week
- side effects occur. You may report side effects to FDA at1-800-FDA-1088
If Pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than directed unless advised by your doctor.
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- fill to top of white section in cap which is marked to indicate the correct dose (17g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use not more than 7 days
- children 16 years ofage or under: ask a doctor
- store at 20o- 25oC (68o-77oF)
- tamper- evident: do not use if foil seal under cap, printed with "SEALED for YOUR PROTECTION" is missing, open or broken
| POLYETHYLENE GLYCOL 3350
polyethylene glycol 3350 powder, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) |
| Registrant - Emcure Pharmaceuticals Limited (916921919) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Heritage Pharma Labs Inc. d/b/a Avet Pharmaceuticals Labs Inc. | 189630168 | ANALYSIS(14539-150) , LABEL(14539-150) , MANUFACTURE(14539-150) , PACK(14539-150) | |