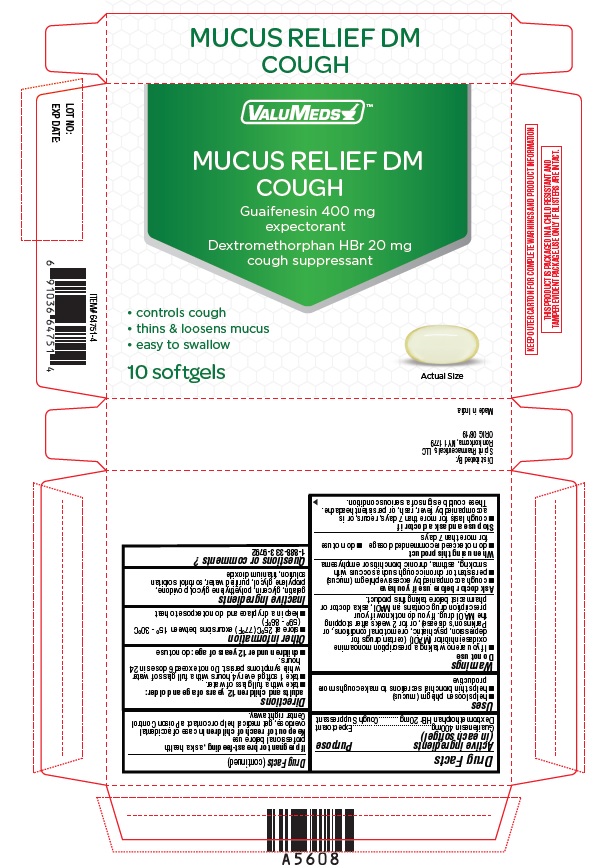
| Active ingredient (in each softgel) | Purposes |
| Guaifenesin 400mg | Expectorant |
| Dextromethorphan HBr 20mg | Cough Suppressant |
Uses
- helps loosen phlegm (mucus)
- helps thin bronchial secretions to make coughs more productive
Warnings
Do not use
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask Doctor before use if you have
- cough accompanied by excessive phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
When using this product
- do not exceed recommended dosage
- do not use for more than 7 days
Stop use and ask a doctor if
- cough lasts for more than 7 days, recurs, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding ask a health professional before use
Keep Out of Reach of Children In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older:
- Take with a full glass of water.
- Take 1 softgel every 4 hours with a full glass of water while symptoms persist. Do not exceed 6 doses in 24 hours.
-
Children under 12 years of age: do not use
Other information
- store at 25°C (77°F) excursions between 15° - 30°C (59° - 86°F)
- keep in a dry place and do not expose to heat
- you may report side effects to 1-888-333-9792
Inactive Ingredients
gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol sorbitan solution, titanium dioxide
Questions or comments?
1-888-333-9792
PRINCIPAL DISPLAY PANEL
Mucus Relief DM
COUGH
GUAIFENESIN 400 mg/EXPECTORANT
DEXTROMETHORPHAN HBr 20 mg/COUGH SUPPRESSANT
DISSOLVES QUICKLY
Controls cough
Thins & loosens mucus